
Never forget a face, a name, or a memory — with this, you can remember it all…
—-Important Message From Our Sponsor—-
Pour this homemade liquid in your ears to stop Alzheimer’s
More than 253,596 people have tried this simple and cheap homemade liquid that improved their brain function and boosted their memory.
Brain doctors cannot believe this dirt-cheap method is better than anything else.
But scientists at the University of Utah confirmed this homemade liquid can quickly reverse memory damage, forgetfulness, difficulty concentrating and confusion.
3-Ingredient Homemade Liquid Can Quickly Improve Alzheimer’s
———-
This is the perfect memory hack for older men to appear young
Multiple lines of evidence converge to implicate NMDA glutamate receptors, and not the AMPA or kainate types, in the process of memory consolidation.
Chemicals that selectively activate the NMDA receptor type, such as piracetam, have consistently been shown to promote learning (Rudel, 1984).
Conversely, the NMDA antagonists ketamine and PCP (phenylcyclohexyl piperidine) are classic amnesiac agents.
Mice genetically engineered to lack the NMDA receptor have impaired memory consolidation (Shimizu, 2000), spatial learning deficits (Tsien, 1996), and the inability to retain thoughts (Cui, 2004) — effects not seen after deleting the other receptor subtypes.
This receptors’ eponymous ligand — N‑methyl‑D‑aspartate (NMDA) — has been shown to promote learning in monkeys, even despite a brain surgery being performed to directly apply the treatment (Dudkin, 1996).
For these reasons, and for some gone unmentioned, the NMDA receptor is now widely considered the one most intimately involved in memory formation.
‘The NMDA receptor appears to play an integral role in memory.’ ―Magnusson, 1998
In addition to being consistently supported through pharmacological experiments, clinical trials, and genetic engineering, the veracity of this proposition is further enforced by studies on long‑term potentiation.
Long‑term potentiation (LTP) is a unique effect that’d been discovered in the late ’60s & early ’70s by Norwegian neurophysiologists (Lømo, 1973).
After applying electrical impulses to nerve tracts using certain frequencies, it was shown that the amplitude detected downstream will increase with time.
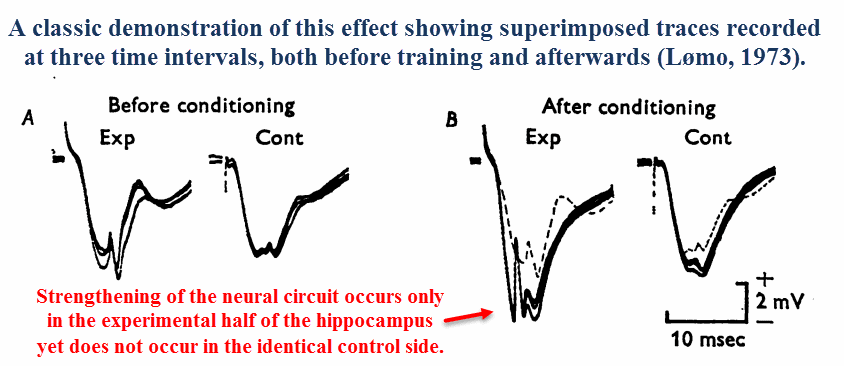
A reduction in propagation time had also been observed — the electrical impulses had progressively arrived sooner and sooner upon each stimulation.
Since this phenomenon implies a “strengthening” effect of neural circuits as a function of use, long‑term potentiation had quickly been adopted as a learning paradigm soon after its discovery.
After considering the plausibility of long‑term potentiation being involved in the learning process, or even being the process itself, it probably shouldn’t be a surprise that NMDA receptors were found involved in this.
Glutamate has been shown to be a requirement for long‑term potentiation as early as 1984, and this effect ONLY occurs through the NMDA receptor:

After noting the many treatments previously shown to inhibit long‑term potentiation are also selective NMDA antagonists, Eric Harris of California set out to investigate the link.
To test this he used five similar molecules differing in chain length, only two of which were selective NMDA antagonists.
On account of the high degree of similarity between the test compounds, his results rule out alternative explanations for this effect:
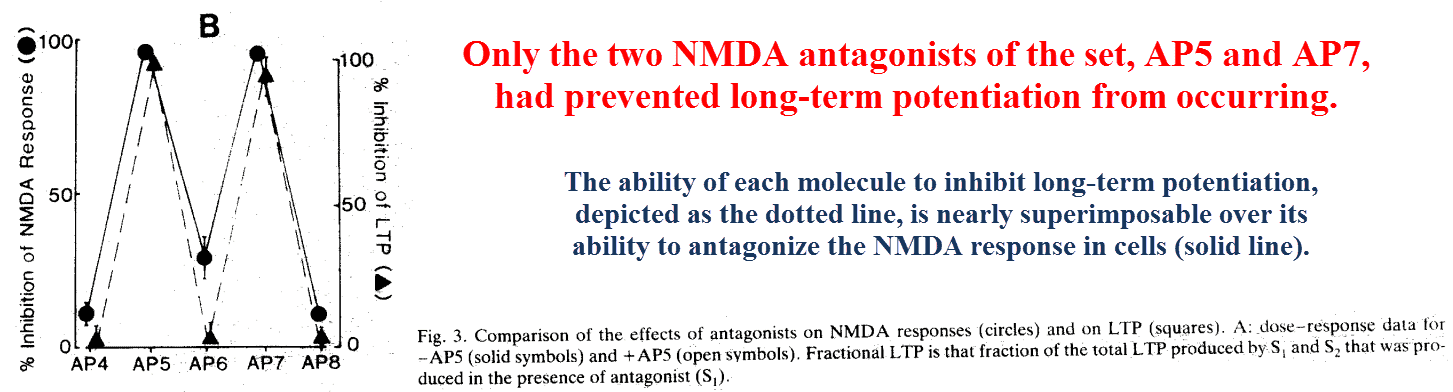
He also showed that kainate and AMPA receptors have different binding profiles for this set of ligands, being unaffected by AP5 through AP7 and only slightly inhibited (~10%) by AP4 and AP8.
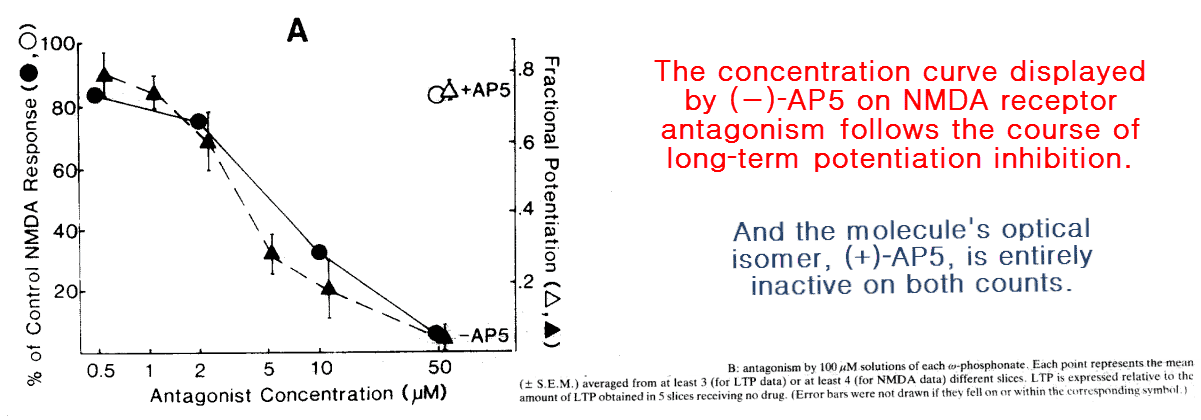
He further solidified his case by using a stereoisomer of AP5, which as a chiral molecule has two identical mirror images.

Optical isomers — a.k.a. Enantiomers — can be thought of as similar to left and right hands, identical when you have a mirror to reflect the image.
A left‑hand glove will of course not fit on the right, and the same can be said about shoes.
He found that only the (−)‑enantiomer prevented the response, the same one that binds the NMDA receptor.
Since enantiomers are chemically identical in every respect besides their interactions with other chiral molecules, such as receptors, this rules out any other conceivable explanation for the effect.
These effects on long‑term potentiation have never been demonstrated to occur through any other receptor subtype (Collingridge, 1987), begging the question of:
“What makes the NMDA receptor special in this regard?”
Although they do all have slightly different expression profiles in the brain (Ritter, 2001), the NMDA receptor differs from the other two in one crucial regard:
Although all three receptor types activate the cell influx of sodium (Na+) and chloride (Cl−) ions, only the NMDA receptor also mediates the ingression of calcium (Ca2+).
‘However, only the channels opened by NMDA receptors are, in addition, highly permeable to Ca2+.’ ― Choi, 1988
Calcium permeability is determined by the subunits comprising the receptors, with the NMDA subtype being the only one composed of high‑conductance NR1 & NR2 subunits (Dingledine, 1999).
The NMDA receptor is also the only type invariably coupled to phospholipase C, an enzyme that liberates inositol triphosphate — a Ca2+ chelator — from the cell membrane.
Although some AMPK variants also couple with this enzyme, and this does confer the ability to mediate Ca2+ influx (Marzoni, 1991), this generally does not occur in AMPK receptors and never in the kainate subtypes.

This ability to mediate calcium ingression translates into enhanced neurite outgrowth, a consistent experimental observation that’s also a theoretical expectation.
This is because calcium has been shown in simple in vitro models to depolymerize both actin and microtubule cytoskeletons, thereby allowing sodium‑mediated expansion and subsequent remodeling after the Ca2+ concentration equilibrates.
‘The net effect of Ca2+ on this family of proteins would appear to be cleavage of the actin network system into filament fragments and formation of new nuclei that would grow in response to further signals to polymerize.’ ―Jamney, 1994
While many proteins are thought to help orchestrate this event, gelsolin and profilin are perhaps the two most involved (Forsher, 1989).
Gelsolin selectively binds calcium in a manner that leads to an alteration in its structure, conferring the protein an ability to depolymerize actin.
Profilin strongly binds actin monomers and keeps them from becoming fibers but will release them if displaced by phosphatidylinositol, now thought as a signal for actin fiber regrowth (Jamney, 1994).
A similar thing happens to the more rigid microtubule cytoskeleton.
Calcium has become so notorious in inhibiting the polymerization of tubulin — the monomer — into microtubules that EGTA is now invariably added when elongating them in vitro.
The Ca2+ inhibition of microtubule growth is a classic effect (Weisenberg, 1972), and the very reason why initial attempts and polymerization had been unsuccessful.
The requirement of GTP for microtubule elongation is also a classical effect, and perhaps why Ca2+–calmodulin phosphodiesterase has also been found to play a role (Marcum, 1978).
‘The cumulative effect of increased Ca²⁺ in the absence of other signals would appear to be a solubilization of the actin cytoskeleton, which would allow for such effects as myosin-based contraction, osmotic expansion, secretion, and preparing the cytoskeleton for reformation in response to a subsequent signal.’ ―Jamney, 1994
And incidentally, the selective Ca2+‑chelating molecule EGTA has been shown to stimulate dendrite growth (Pearce, 1987).
This very same molecule also prevents long‑term potentiation (Lynch, 1983), likely by canceling the “remodeling” signal initiated by Ca2+ influx.
Although calcium influx can occur everywhere throughout the neuron when high amounts of glutamate are artificially applied, internal remodeling is thought to only occur naturally in confined regions of dendrites.
This is because glutamate concentrations only get high enough in areas directly adjacent to axons, or the cells which release it across synapses.
For this reason you could only expect dendrites in certain areas to be responsive to glutamate in this way, and only in those containing NMDA receptors.
The influx of sodium (Na⁺) and chloride (Cl−) ions, also mediated through NMDA receptors, are also expected to play a role.
These two ions have been shown to induce swelling, and should the new cytoskeleton be rebuilt in this state the “swollen form” would become fixed.
The expectations drawn from in vitro studies have played out…
The NMDA glutamate receptor specifically, and no other, has been shown to induce neurite outgrowth when activated.
This study had been undertaken after previous reports of calcium being correlated with neurite outgrowth.
So on account of NMDA mediated Ca2+ influx also being known at the time, the idea that glutamate could do this had been a rational theoretical expectation.
‘Cells that were rapidly extending showed high Ca2+ levels in the regions of growth. […] In active growth cones distant from the soma, Ca2+ levels exceeded 200 nM, whereas the soma levels were in the 60-80 nM range. […] The results show that high Ca2+ levels are at least a correlate of extension in CNS cells and that under some conditions the region of high calcium can be localized to a small part of the cell.’ ―Connor, 1986
He had started out using kynurenate, a broad spectrum glutamate inhibitor that antagonizes every receptor subtype.
Kynurenate is not to be confused with kainate, the small molecule found in seaweed that selectively activates a glutamate receptor subtype.

Kynurenate is a natural tryptophan metabolite formed through the kynurenine pathway.
He’d found that kynurenate would substantially inhibit the number of cells demonstrating neurites — a somewhat generic term used to describe cell processes in general.
Neurites can be either axons or dendrites, yet discerning the particular type is not always necessary and is more difficult besides.
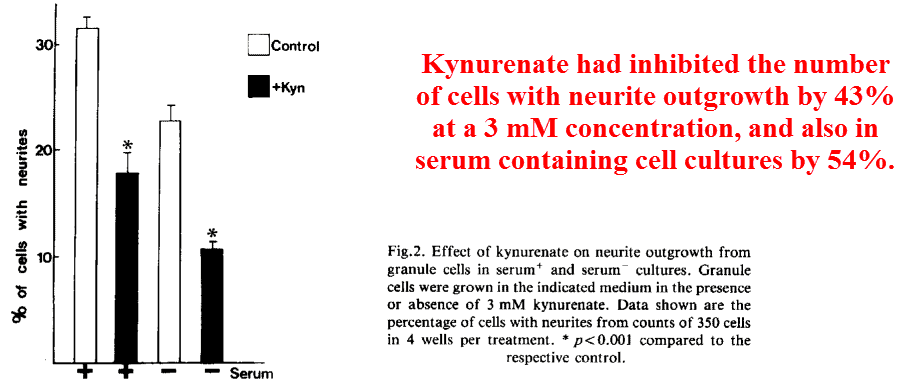
Yet because kynurenate is a broad spectrum inhibitor of every glutamate receptor, this finding means relatively little in terms of elucidating the exact target.
But after noting that only the NMDA subtype mediates calcium influx, and that Ca2+ is correlated with neurite outgrowth, he decided to see what would happen after using aminophosphonovalerate (APV).
‘However, a major difference between NMDA and non-NMDA receptor agonists is the specific ability of NMDA to open channels which allow Ca2+ influx and a rise in cytosolic free Ca2+.’ ―Pearce
Aminophosphonovalerate (APV) is a classic antagonist of NMDA receptors and is synonymous with (−)‑AP5, the chemical found active in inducing long‑term potentiation in the study above.
Confirming his suspicion, he found that APV was every bit as effective as kynurenate in inhibiting neurite outgrowth. This finding suggests that the NMDA receptor is primarily responsible for this effect.
And also, he found that glutamate could reverse this inhibition in a dose‑dependent manner:
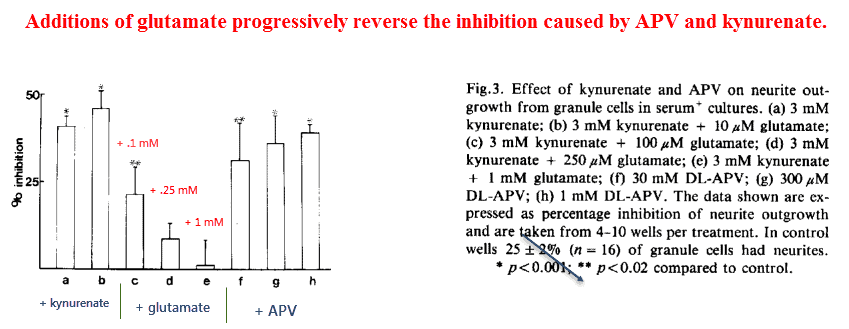
Although this proves the NMDA receptor is necessary for neurite outgrowth it doesn’t prove it’s sufficient.
The addition of NMDA itself, on the other hand, would leave little doubt…
Only 30μM of NMDA had completely reversed the 43% inhibition caused by 30μM aminophosphonovalerate (APV).
The finding that a classic agonist of the NMDA receptor could abolish the effects of a highly specific antagonist of the same, and at the same concentration, is highly suggestive that only the NMDA subtype is primary.
After considering the fact that a specific NMDA ligand (APV) had inhibited as well as a general one (kynurenate), you are left with the conclusion that the NMDA receptor is responsible for practically all of glutamate’s neurotrophic effects.
Activators of the NMDA receptor are also the same treatments shown to clinically promote learning, and also to inhibit it in the case of ketamine and PCP (phenylcyclohexyl piperidine, angel dust).
‘In general, glutamatergic ‘treatments’ improve learning and memory, and conversely, ‘treatments’ that decrease glutamatergic function impair learning and memory.’ ―D’Souza, 1995
Glycine and D‑serine also selectively activate this receptor, both acting allosterically to potentiate glutamate’s action (Mothet, 2000).
Having at least one of these is actually required for NMDA to have effect, yet there’s some indication that D‑serine is the more potent (Shleper, 2005).
The D‑isomer of L‑serine is actually formed naturally in the brain by serine racemase, an enzyme ubiquitously expressed throughout the body (Xia, 2004).
It has been shown that the peripherally administered L‑serine will, in fact, convert into D‑serine in the brain & cerebrospinal fluid as expected (Hashimoto, 2000).
And moreover, D‑serine appears better than glycine for yet another reason…
This amino acid is more selective than glycine on the NMDA receptor, it’s only known target, and this is because glycine displays activity through its own receptors (GlyR).
So besides piracetam and its related synthetics, the only apparent way to SELECTIVELY activate the NMDA receptor is by consuming either D‑ or L‑serine.
This is highly fortunate because serine is safe, affordable, and freely available online.
Serine also has other functions.
Besides being an amino acid used to construct proteins, L‑serine is actually a significant precursor for endogenous acetylcholine synthesis (Bremer pathway).
‘A reasonable hypothesis, therefore, is that when the Mg2+ block of NMDA channels is reduced, Ca2+ enters the cell via these channels and triggers the processes leading to long‑term potentiation.’ ―Collingridge
So whether you use piracetam or serine to accomplish this, enhancing the consolidation of memory should be achievable.
Sounds like a bold statement, for sure, but the scientific evidence is really unanimous in this regard…
Glutamate stimulates neurite outgrowth, long‑term potentiation, and learning through the NMDA receptor in the hippocampus.
There are only a few treatments available which selectively activate this target.
The brain region most characteristically enriched in NMDA receptors is the hippocampus (Monyer, 1994).
This area is responsible for integrating incoming VISUAL information, and for this reason you might expect only certain types of learning and memory enhancement.
Auditory learning is associated more with the nucleus basalis in the basal forebrain (Weinberger, 2003), an area heavily innervated with acetylcholine axons.
It could be worth mentioning that NMDA receptor knockout mice show selective reductions in SPATIAL learning (Tsien, 1996), with nonspatial types apparently unaffected.
‘Also, these mice show impaired spatial memory (measured in the hidden-platform version of the Morris water maze) but display good performance in nonspatial learning tasks.’ ―Tsien, 1996
In mice ‘spatial learning’ means good performance on the Morris water maze with a hidden platform, yet in humans this could translate into: reading, looking, watching, and perhaps even playing tennis.
But because most people “hear” what they read, in a sense, a role for acetylcholine in reading shouldn’t be discounted.
Serine is expected to potentiate both neurotransmitters involved in learning, glutamate through its D‑isomer and acetylcholine through its L‑isomer.
So NMDA agonists certainly have a place among nootropics and are of no less importance, as a class, than cholinergic and adrenergic agents.
‘These results indicate that endogenous glutamate, possibly released by granule cells themselves, stimulated neurite outgrowth through activation of the NMDA class of glutamate receptors. Activation of NMDA receptors on developing neurons may be an important mechanism for the regulation of neuronal growth and differentiation.’ ―Pearce, 1987
—-Important Message From Our Sponsor—-
My mom came out of Alzheimer’s — this REVERSED it
This breakthrough discovery is causing riots among brain doctors who have always insisted that loss of the mind is a one way street.
Well, I’m here to show you what worked for me and my mom. The doctors were completely wrong.

———-

Lømo, Terje. "Long‐lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path." The Journal of physiology (1973) http://www.neuroscience.ucsf.edu/neurograd/files/NS230wi08/2-27-08%20Bliss%20and%20Lomo.pdf
Harris, Eric. "Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors." Brain research (1984) https://www.sciencedirect.com/science/article/pii/0006899384902750
Pearce, Ian. "Glutamate acting on NMDA receptors stimulates neurite outgrowth from cerebellar granule cells." Febs Letters (1987) https://core.ac.uk/download/pdf/82093221.pdf
Janmey, Paul. "Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly." Annual review of physiology (1994) https://www.annualreviews.org/doi/pdf/10.1146/annurev.ph.56.030194.001125
[4]Collingridge, G. L. "NMDA receptors-their role in long-term potentiation." Trends in neurosciences (1987) https://www.cell.com/trends/neurosciences/pdf/0166-2236(87)90175-5.pdf
Dudkin, K. N. "Neurophysiological correlates of improvements in cognitive characteristics in monkeys during modification of NMDA-ergic structures of the prefrontal cortex." Neuroscience and behavioral physiology (1996) https://link.springer.com/article/10.1007/BF02359497
