
Use one or all 4 minerals, and leave the chemicals behind
—-Important Message From Our Sponsor—-
Try this MD’s surprising erections solution — just eat this one food before 9am

Hi, I’m Dr. Khan, and by eating this food every morning, men are seeing their erections restored to full strength…
Their wives and girlfriends are enjoying incredible sexual satisfaction…
And their confidence is through the roof.
No drugs, no pumps, nothing weird you have to buy — this a simple, NATURAL way to boost your erections quality.
I’m telling all my male patients about this because it actually works.
Just eat this food before 9am for fully restored erections and more lasting power
———-
These 4 minerals prevent blood sugar problems in men
Despite what the American Medical Association would have us believe, many Americans are now fully aware that blood sugar problems have an underlying nutritional basis.
The majority of scientists are also well aware of this, yet due to the complexity of insulin signalling, the exact mechanism(s) remains difficult to pinpoint.
Instead of having just one cause, there appears to be many, each acting in some way to interfere with proper signalling and/or enzymatic energy production.
Minerals stand at the forefront of the factors consistently found lower in diabetics, with many having been shown to improve or — as in the case of iron (Fe3+) — exacerbate the condition.
The minerals generally regarded as being the most helpful for diabetics are magnesium (Mg2+), chromium (Cr3+), vanadium (V2+), and manganese (Mn2+), yet they all have varying degrees of efficacy and controversial modes of action.
The reason why magnesium improves diabetes is perhaps the most obscure of them all, despite the fact that it has the most evidence in support that it does.
Perhaps it’s sufficient to say that magnesium is an essential cofactor of over 300 enzymes, four of which are involved in the Krebs cycle…
And that insulin has been shown to pull Mg2+ specifically into cells upon binding its receptor.
Chromium has the most well‑defined antidiabetic role of them all, despite being currently listed as being nonessential.
After being discovered as a component of the “glucose tolerance factor” extracted from brewer’s yeast in the ’50s, it was eventually shown to have a specific blood carrier protein called chromodulin having high affinity to the insulin receptor (Kd = 250 pM).
It’s been shown that chromodulin-Cr3+ complex binds specifically to the insulin receptor, it’s only known biological target, in extremely low concentrations.
This chromodulin binding greatly increases receptor kinase activity and ONLY in the presence of insulin.
Vanadium is somewhat less effective than chromium and magnesium.
Although many researchers are convinced that it increases insulin signalling by inhibiting PTP1B…
The high concentrations in which it does so in vitro simply cannot be reconciled with the low doses shown effective in vivo.
A more realistic appraisal suggests instead that it doesn’t act through insulin signalling but through enhancing thyroid hormone, the main effect observed in vanadium animal studies.
Many lines of evidence add up to suggest that vanadium is the unrealized cofactor of thyroid peroxidase‑2, an otherwise inactive isoform.
Vanadium then is better viewed as a “prothyroid” mineral than an “antidiabetic” one.
This takes us to manganese, an antidiabetic mineral similar to magnesium in many respects.
These two are the only antidiabetic minerals also considered essential, so even if you don’t have diabetes or metabolic syndrome you’d still want to ensure adequate levels of both Mg2+ and Mn2+.
Manganese is also similar to magnesium in that it has a multitude of well‑defined cellular targets that can be argued to contribute, at least to some degree, towards its antidiabetic effects.
This has led to some ambiguity over their precise modes of action, yet regardless there’s certainly a large amount of evidence to prove they work:

This case report describes an 18 year‑old diabetic male presenting with hyperglycemia alternating with hypoglycemia.
Insulin was found to have little effect during the hyperglycemic periods, so upon the patient’s insistence the doctors had allowed him to try his home remedy…
This had led to the startling realization that lucerne, an extract of alfalfa, had a greater ability to lower blood glucose than insulin itself.
After noting that alfalfa contains 45.5 mg⁄kg manganese, “a significant quantity,” Doctor Rubenstein had then tested the effects of oral Mn2Cl.
It was found that just 5mg of Mn2+ could decrease blood glucose more effectively than both insulin and the alfalfa extract:
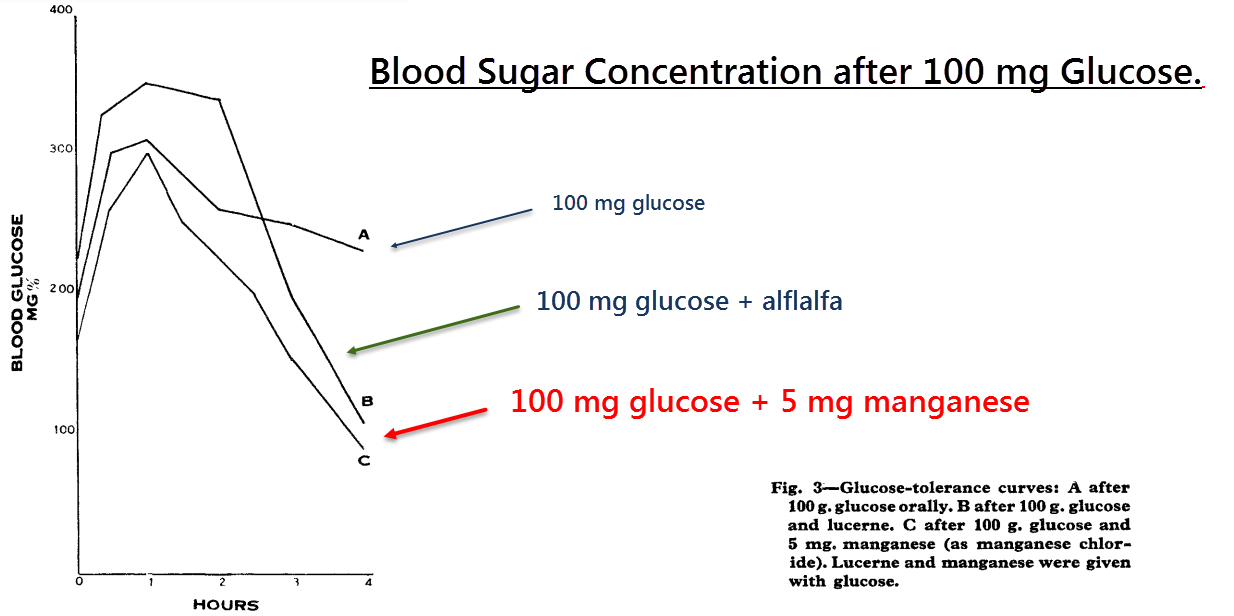
Not bad for a mineral. This demonstrates that the antidiabetic effect of alfalfa, a plant which also contains chiro‑inositol, can be at least partially explained by manganese.
It was then found that the patient had an unusually high Mn2+ excretion rate, thus logically explaining why manganese had a lesser effect on the other members of his family.
“Oral manganese controlled the diabetes no less satisfactorily than soluble insulin.” ―Rubenstein, 1962
So manganese certainly appears to have an important role in insulin signalling, at least in Mn2+‑deficient people.
Males could be expected to have lower amounts of manganese than females in general because they have higher iron concentrations, an element that competes with Mg2+ for uptake and distribution.
It’s been shown that DCT1, the main iron uptake channel found in the intestine, is also responsible for the absorption of manganese.
“In conclusion, iron treatment in both the presence and the absence of serum resulted in a decreased uptake of iron and manganese […]” ―Tallvist, 2000
This ion channel is negatively regulated by iron, meaning that when consuming high amounts of iron without manganese…
As in the case of refined and enriched rice, wheat, and corn…
A downregulation of DMT1 would incur and a reduction of Mn2+ absorption would follow.
In other words: High amounts of inorganic iron will decrease its own uptake as a protection mechanism and, incidentally, that of manganese in the process.
It’s known that heme iron largely bypasses this regulatory process because it doesn’t activate intestinal iron‑responsive proteins.
This is perhaps the reason why manganese deficiency is only correlated with inorganic iron intake.
This is a good reason why enriched foods should be avoided, and also one possible explanation for why iron overload can cause insulin resistance.
After the case report published in 1962, the importance of manganese in glucose control had then begun to be investigated in animals.
A follow‑up study had shown that manganese‑deficient guinea pigs fed Mn2+‑deficient diets had diabetic blood glucose curves.
This was reversible with manganese supplementation, and similar findings were later shown in rats.
More detailed studies had then found reduced insulin mRNA concentrations in Mn2+‑deficient pancreatic β‑cells.
Although this was a step in the right direction, the precise mechanism still remains a mystery.
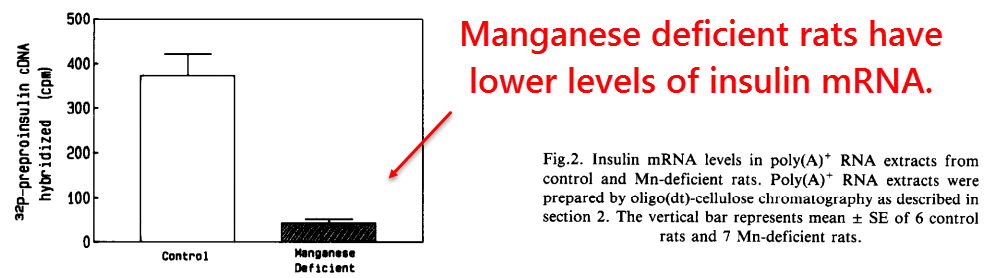
What is known for sure, however, is that insulin mRNA contains hair‑pin bends similar to iron‑response elements.
It is also known that rats with iron overload have decreased insulin mRNA in pancreatic β‑cells.
For these reasons, it wouldn’t seem too unreasonable to suggest that insulin mRNA is regulated by iron status through iron‑response proteins (IRPs) — which are also sensitive to redox status.
Newer studies help shed light on this matter:

This study had been conducted by a group in Utah in response to earlier findings using iron‑overloaded rats showing…
157% increased iron, 38% decreased manganese, 61% increased lipid peroxidation, and 27 to 51% decreased mitochondrial energy levels.
In another of their previous studies on iron‑overloaded rats, they’d also found lower β‑cell insulin mRNA and reduced insulin output.
This had led to the discovery that these diabetic changes were partially corrected by manganese supplementation, thus finally confirming decades‑old research:
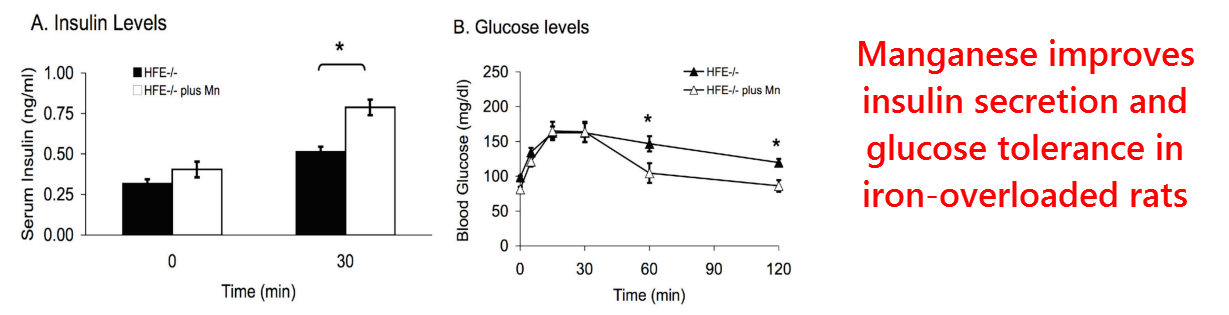
So in the aim of finding out why this occurs, a third study had been planned this time to test the effects of manganese specifically on normal rats.
They had also used a high‑fat diet to induce insulin resistance in some groups. Since this high‑fat diet consisted of lard and soybean oil, it could be considered a high‑PUFA diet.
They had focused on the enzyme Mn2+‑superoxide dismutase because they’d found this to increase in previous studies.
Superoxide dismutase is an extremely important enzyme and comes in two forms, a mitochondrial Mn2+‑dependent one and a cytosolic Cu2+‑dependent one.
This enzyme catalyzes the removal of superoxide (•O2−) — a dangerous free radical — by transforming it safely back into oxygen (O2).
So they found, as expected, a 70% increase in mitochondrial manganese and a 60% increase in superoxide dismutase activity.
They also reported significant increases of the manganese content of superoxide dismutase, thus showing that some enzyme normally exists in its inactive apo‑ form.
Similar findings were found in their pancreatic β‑cells, along with a 59% increase of secreted insulin in the manganese‑supplemented group.
This corresponded to an increase in glucose tolerance good enough to confer normalcy to rats consuming a high‑PUFA diet:
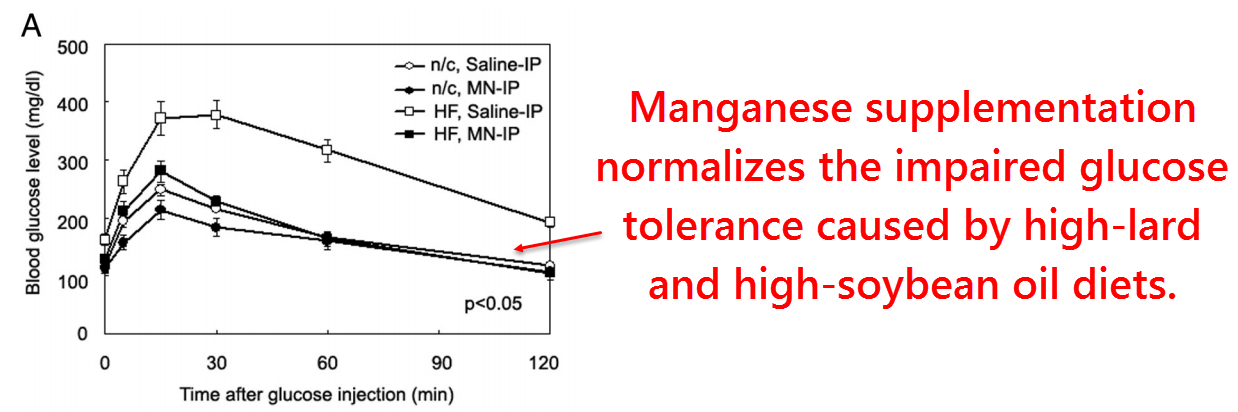
These are interesting findings to say the least, yet it doesn’t necessarily prove that this effect of manganese is dependent upon superoxide dismutase.
Manganese has many cellular targets and also increases the enzyme activities of arginase, prolidase, enolase, galactosyltransferase, protein phosphatase, etc…
Yet regardless of the other Mn2+‑dependent enzymes, other evidence suggests that superoxide dismutase plays a significant role.
For example, the overexpression of superoxide dismutase has been shown by other researchers to increase glucose uptake in normal rats and those fed a high‑PUFA diet.
In addition, Japanese researchers have found in a prospective trial that subjects having the valine16 variant of Mn2+‑superoxide dismutase carry double the risk of developing diabetes than those having the alanine16 variant.
It’s even been proposed that superoxide itself is fundamental to insulin resistance as it lies at the nexus of many forms of insulin resistance.
It’s been shown that the insulin resistance caused by dexamethasone, TNF‑α, antimycin A, and high glucose all share a common denominator in causing increased mitochondrial superoxide.
Moreover, all of these forms of insulin resistance can be improved and restored to normalcy through the overexpression of Mg2+‑superoxide dismutase.
These findings have led the author to suggest that superoxide is a “turn off signal” for the mitochondria, lowering energy production before excessive damage can occur.
“In summary, the fact that mitochondrial •O2− is upstream of insulin resistance is of major significance suggesting that insulin resistance may be part of the antioxidant defense mechanism to protect cells from further oxidative damage.” ―Hoehn, 2009
This explanation is quite intuitive, and a good way to prevent the cell from destroying itself with free radicals.
A likely target for this “mitochondrial shut‑off” signal could be aconitase, an enzyme with a catalytic iron-sulfur cluster shown to be inactivated by low concentrations of superoxide.
Aconitase converts citrate into iso‑citrate, hence making it a key component of the Krebs cycle.
So the antidiabetic effect of Mn2+ can perhaps be explained entirely through superoxide dismutase, yet it has a few other cellular targets that deserve mention:
Manganese has been shown to recapitulate many of insulin’s effects such as activating glycogen synthase phosphorylase, cAMP phosphodiesterase, and insulin receptor autophosphorylation when complexed with ATP.
“Mn2+ was most stimulatory, Mg2+ was next, and Ca2+ was least effective. The stimulatory effects were enhanced by insulin.” ―Ueda, 1984
Manganese also appears to be chelated specifically by chiro‑inositol, a component of the “insulin mediator” on cell membranes that’s released in response to insulin and then internalized.
For these reasons, and because chiro‑inositol and Mn2+ synergize with each other, it could be supposed that the main function of an “insulin mediator” is to chelate manganese and bring it inside the cell.
“Isolation of putative insulin second messengers from beef liver yielded a pseudo-disaccharide consisting of pinitol (3‑O-methyl‑ᴅ‑chiro‑inositol) α‑1,4 linked to galactosamine chelated with Mn2+ (called INS2).” ―Braughtigan, 2005
So manganese has a lot going for it, and should help most people control blood sugar while lowering free radicals in the process.
Manganese is also a component of galactosyltransferase, an enzyme needed to make glycosaminoglycans necessary for skin volume (hyaluronan) and bone mineralization (chondroitin‑4‑sulfate).
This is why it’s also found in anti‑arthritis supplements, to produce more shock‑absorbing hyaluronan in the synovial fluid.
—-Important Message—-
Why does this special breakfast help reverse blood sugar problems?

One of the main ingredients in my special blood sugar breakfast is vitamin K2.
And vitamin K2 raises testosterone in men…and it also has another big health benefit…
It lowers blood sugar naturally, and even reverses symptoms in diabetics and prediabetics.
So now you can raise testosterone, lower harmful estrogen, and get normal blood sugar just by drinking this deliciously simple, easy-to-make breakfast loaded with vitamin K2…
And the benefits will be obvious when you wake up with impressive morning wood…
Go here for this special blood sugar lowering breakfast for men — raises T and restores rockiness
———-

Rubenstein, A. H. "Manganese-induced hypoglycaemia." Lancet (1962) https://www.cabdirect.org/cabdirect/abstract/19631404967
Baly, Deborah L. "Mechanism of decreased insulinogenesis in manganese‐deficient rats: Decreased insulin mRNA levels." FEBS letters (1988) https://core.ac.uk/download/pdf/82739873.pdf
Lee, Soh-Hyun. "Manganese supplementation protects against diet-induced diabetes in wild type mice by enhancing insulin secretion." Endocrinology (2013) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578995/
Jouihan, Hani A. "Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis." Molecular medicine (2008) https://www.researchgate.net/profile/Paul_Cobine/publication/5533813_Iron-Mediated_Inhibition_of_Mitochondrial_Manganese_Uptake_Mediates_Mitochondrial_Dysfunction_in_a_Mouse_Model_of_Hemochromatosis/links/00463522202c88a769000000/Iron-Mediated-Inhibition-of-Mitochondrial-Manganese-Uptake-Mediates-Mitochondrial-Dysfunction-in-a-Mouse-Model-of-Hemochromatosis.pdf
Hoehn, Kyle L. "Insulin resistance is a cellular antioxidant defense mechanism." Proceedings of the National Academy of Sciences (2009) https://www.jbc.org/content/268/30/22369.full.pdf
