
[cmamad id=”19959″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
Why many men believe soybeans are part of a very real conspiracy against manhood
——Important Message—–
The secret to male confidence, stamina, and muscles (even better erections)
Ray was married three times. The latest wife filed for divorce and Ray was devastated. “I thought she was the one. But I can’t blame her.”
Ray realized that he had suffered from low confidence for his entire life.
“Women are attracted to very confident men,” he says. “I needed to become more confident.”
Ray then found out, through my teaching, how testosterone makes men more confident.
He got his T checked, and it was very low. So I showed him how to raise his T – from 302 to 907 in his case.
If you are a man who wants to have a great life, you need to have high T.
High testosterone leads to these benefits:
- Makes you super confident
- You don’t care anymore about stupid little things (and everything is a stupid little thing)
- It gives you back your strong drive
- You enjoy great sexual stamina
- You build muscles even if you don’t work out
- Belly fat drops off all the time even though you eat whatever you want
Here’s the secret Ray used to raise his testosterone from 302 to 907 in less than 5 weeks
————–
The “soy boy” conspiracy theory
Over the years, there’s been talk about “plant polyphenols” having estrogenic effects, sometimes with the implication that all of them do.
This is not true. Only a few flavonoids have estrogenic effects.
And there is at least one that is a powerful anti-estrogen.
“In conclusion, we identified baicalein, a naturally occurring flavone, as an anti-estrogen.”
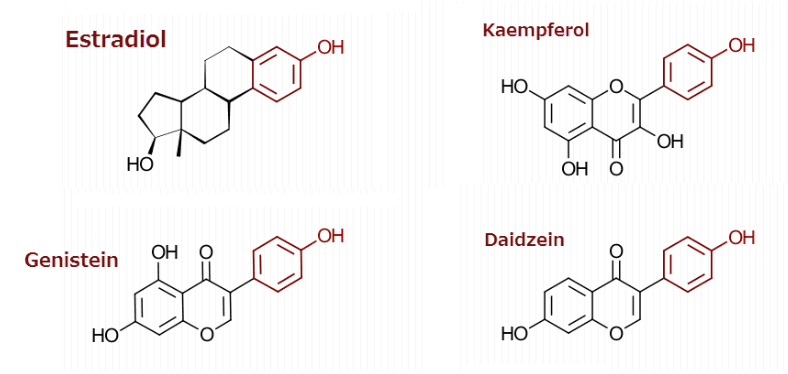
Only kaempferol, genistein, and daidzein have significant affinity for the estrogen receptor alpha (ERα).
Genistein and daidzein are found in soybeans.
Kaempferol is somewhat particular to strawberries.
That’s basically it… No other flavonoid polyphenols will bind the estrogen receptor at physiological concentrations, much less activate it:
The soy isoflavone genistein is about ten times more potent than its nearest competitors (daidzein and kaempferol)…
And that is the best reason for men to avoid soy.
Because women have more estradiol available for inhibition, genistein can be inhibitory for them.
Because of genistein’s strong binding affinity to estrogen receptors, it can compete with the more active estradiol in female cells that have high amounts.
[cmamad id=”19960″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
Yet in males, genistein absolutely has estrogenic activity.
Soy should be avoided by all rational men, yet women and trans women may find it useful.
All three estrogenic flavonoids have one thing in common:
They have a hydroxy group on carbon #4 of the B-ring, flanked by nothing else in every case.
They need this configuration to mimic estrogen:
Dozens of flavonoids have a 4-hydroxy group, yet most of them also have adjacent groups that will prevent it from binding to estrogen receptors.
Many flavonoids, such as baicalein, don’t have a 4-hydroxy group at all.
Baicalein has absolutely nothing on its B-ring, and it is a flavone besides – not an iso-flavone like genistein and daidzein:
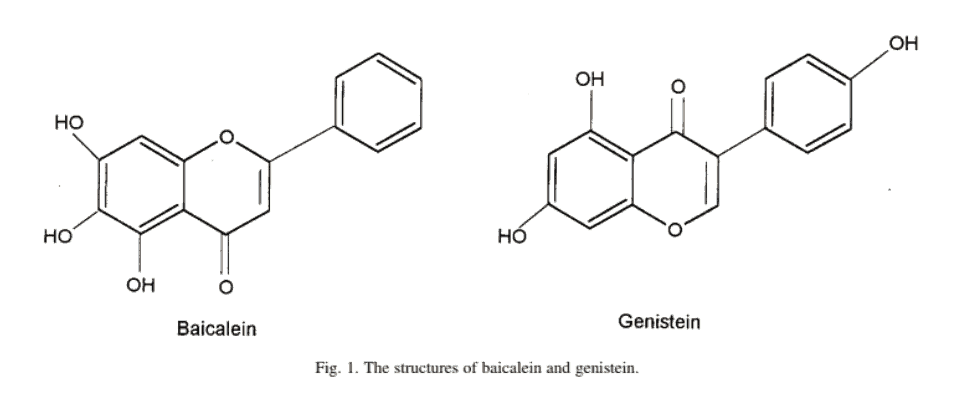
Its inability to interact directly with estrogen receptor alpha (ERα) makes its action seem perplexing.
How exactly does baicalein inhibit ERα transactivation without actually binding to it?
And it doesn’t inhibit aromatase either.
The answer may surprise you…
It’s the same reason why baicalein is also the best anti-cancer flavonoid in general.
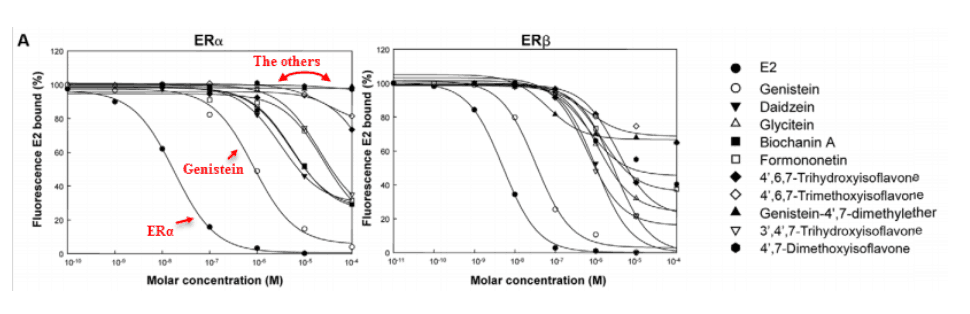
This study proved the anti-estrogenic activity of baicalein in the most direct way possible.
These scientists injected human cells with short DNA strands that encode for luciferase enzymes, which can then produce fluorescence.
Since these DNA strands all had ERα response elements (such as GGTCA and TGACC), they could only be transcribed through ERα.
Hence, in this study: Estrogen receptor alpha activity is directly proportional to fluorescence.
The gene that encodes the prolactin receptor also has an ERα response element…
So the curves in the graph below are also proportional to man-boob size!
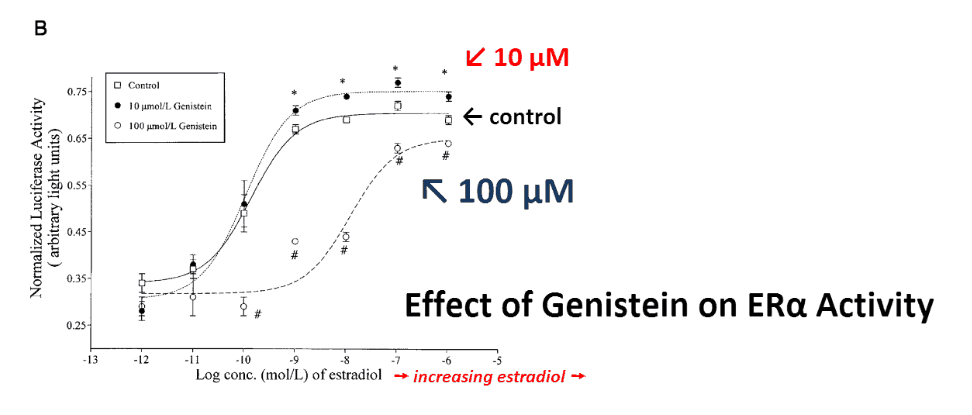
As expected, soy genistein was estrogenic at low concentrations and anti-estrogenic at higher concentrations.
But the higher 100 μM concentration used above cannot realistically be achieved through oral consumption.
Yet a concentration of 10 μM is achievable for most flavonoids, and in many organs.
And this is true for baicalein…
This flavone progressively inhibited ERα activity in concentrations of 10, 50, and 100 μM and it is safe to ingest:
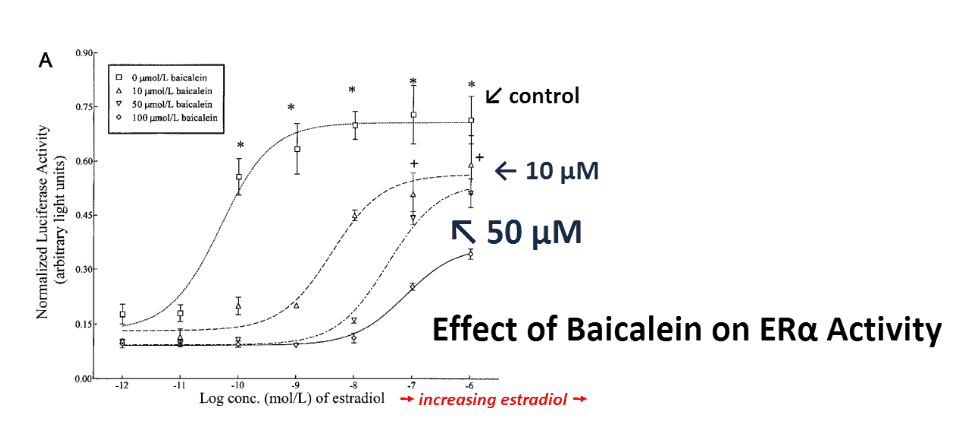
Who would have guessed the most potent anti-estrogen flavonoid would come from a flower?
And a purple one at that? (Scutellaria baicalensis, AKA Baikal skullcap, an herb used in Chinese medicine).
It’s also in thyme.
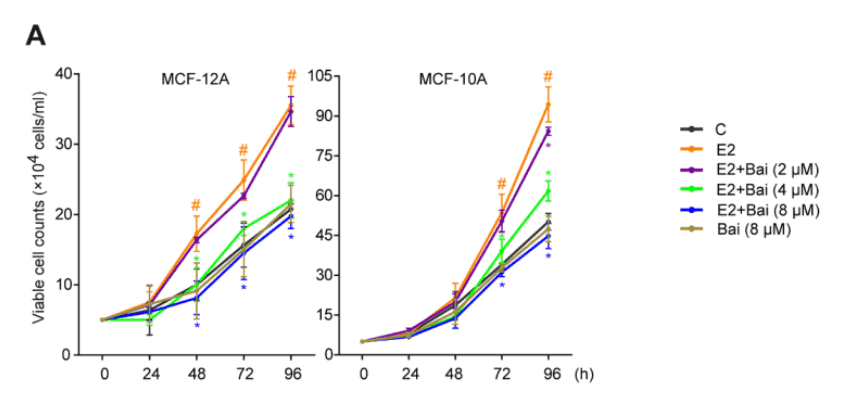
These effects are very consistent and fully corroborated by other studies.
Baicalein is officially a potent anti-estrogen.
The graphs below show it incrementally inhibiting estrogen-induced growth throughout the low micromolar range (1–8 μM):
But still, how does baicalein inhibit ERα activity without directly binding to it, or through inhibiting aromatase?
Well, that happens to be through baicalein’s classic function.
Consistently, studies show that baicalein is the most potent glyoxalase I (GLOI) inhibitor among the entire flavonoid class.
“In this study, 16 flavonoids were examined, and only baicalein…is identified as a potent in vitro GLOI inhibitor.”
Baicalein is second only to lapachol and beta-lapachone among all plant compounds in glyoxalase inhibition.
(Both of those come from the lapacho tree and have anti-cancer and anti-inflammatory properties.)
This means that baicalein can increase intracellular concentrations of methylglyoxal…
Methylglyoxal inhibits and reverses cancer cells without damaging normal cells.
But nobody knew how that actually worked until just recently.
Paul Thornalley proved in 2007 that methylglyoxal can modify transcription factors at arginine side-chains thereby affecting gene expression:
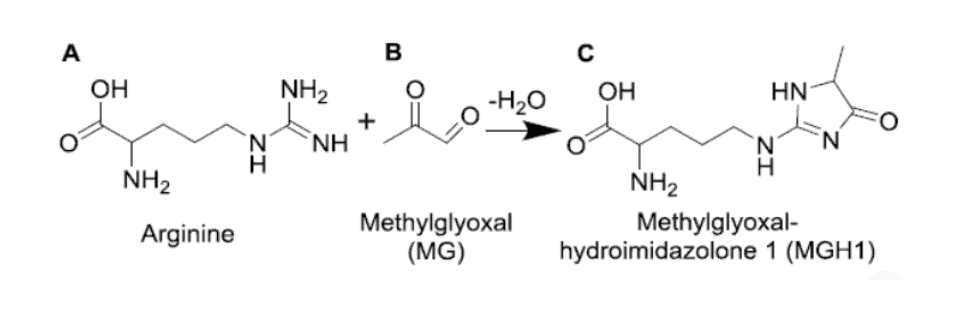
Estrogen receptors are technically transcription factors – they also have an arginine side-chain in the binding pocket.
In the estrogen receptor alpha: Arginine-394 lies directly across from the 3-hydroxy group of estradiol, analogous to the 4-hydroxy group, the soy isoflavones:
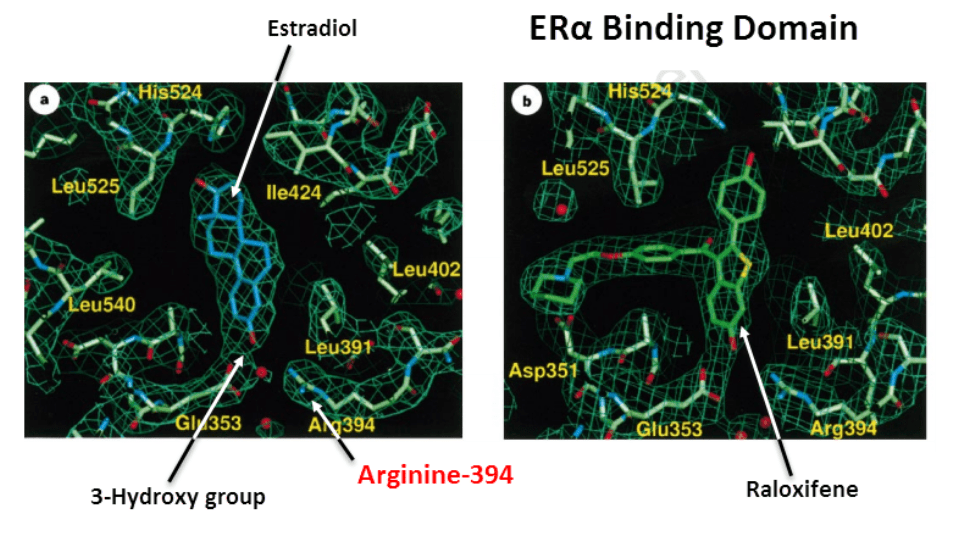
The conversion of arginine-394 to hydro-imidazolone, via methylglyoxal, would certainly inhibit estrogen binding.
Mutation studies reveal that this arginine is necessary for receptor activity.
The positively charged arginine is needed to bind the negatively polarized 3-hydroxy group of estradiol.
“In contrast, baicalein significantly decreased the estradiol-induced nuclear translocation of estrogen receptor alpha.”
The enzymes glyoxalase I and glyoxalase II and glutathione continuously keep methylglyoxal concentrations in check.
But methyl glyoxylate concentrations can be increased by using natural glyoxalase inhibitors such as baicalein, lapachol, and beta-lapachone.
Science is beginning to understand the mechanisms underlying the classical effects of methylglyoxal.
And they involve the selective modification of arginine side-chains on transcription factors and enzymes.
This is a natural process.
And it appears to be the primary way the cell quickly responds to changing glucose/fructose metabolism.
“…treatment with estradiol resulted in the obvious nuclear localization of estrogen receptor alpha…the nuclear localization of ERα was inhibited in the presence of baicalein.”
—-Important Message—-
My two-step size system
You may or may not know that the penis is made up of three chambers or columns of tissue.
The blood flow to these three chambers is often blocked. This restricts blood flow.
That is not good if you want to have great sex.
And it affects size too! Penis size is dictated by how much blood the two bigger chambers can hold.
To make your penis bigger, you need to achieve one thing: get these chambers to take more blood. Here’s where it gets weird.
——–

https://www.tandfonline.com/doi/pdf/10.1080/00021369.1986.10867521
Po, Lai See. "Baicalein and genistein display differential actions on estrogen receptor (ER) transactivation and apoptosis in MCF-7 cells." Cancer letters (2002)
https://www.sciencedirect.com/science/article/pii/S0304383502003555
Xu, Di Pan. "Baicalein has protective effects on the 17β-estradiol-induced transformation of breast epithelial cells." Oncotarget (2017)
https://www.researchgate.net/profile/Yan_Chen54/publication/312050762_Baicalein_has_protective_effects_on_the_17b-estradiol-induced_transformation_of_breast_epithelial_cells/links/58a17c5945851598bab88ba7/Baicalein-has-protective-effects-on-the-17b-estradiol-induced-transformation-of-breast-epithelial-cells.pdf
Zhang, Hong. "In Vitro Inhibition of Glyoxalase I by Flavonoids: New Insights from Crystallographic Analysis." Current topics in medicinal chemistry (2016)
https://www.researchgate.net/profile/Xiaopeng_Hu/publication/281057604_In_Vitro_Inhibition_of_Glyoxalase_I_by_Flavonoids_New_Insights_from_Crystallographic_Analysis/links/5854425808ae77ec37045b3a/In-Vitro-Inhibition-of-Glyoxalase-I-by-Flavonoids-New-Insights-from-Crystallographic-Analysis.pdf

Leave a Reply