
This is a very interesting newsletter.
We’re connecting the dots between jaundice, carbon monoxide, and excessive iron.
And we’re going to have a recommendation that can vastly improve your health.
As usual.
When we brought our baby home from the hospital, it had jaundice.
It had a little bit of a yellow color, and we had to monitor the baby carefully.
Jaundice is caused by an excess amount of the blood ingredient known as heme.
When a baby is born, a lot of that heme has to be gotten rid of.
As heme is broken down, at first it turns into bilirubin.
And bilirubin gives a jaundiced baby, or jaundiced adult, a yellow color.
 I promised we’d come back to the importance of heme, bilirubin, and carbon monoxide.
I promised we’d come back to the importance of heme, bilirubin, and carbon monoxide.
So let’s do it right now.
Let’s look at this review about a compound that most of us have been trained to see as a deadly poison.
Carbon monoxide.
It turns out that carbon monoxide is naturally produced in the body and is an important gaseous messenger.
In a way, health and medicine consist of our progressively undermining previously held beliefs.
New discoveries give us a better understanding of the way our bodies work.
And this often means disproving what we thought of as fact before a particular study.
We used to believe that carbon dioxide was a waste product.
But now we know carbon dioxide is one of the most important substances in our body.
It’s not a waste product but a vital ingredient in every cell.
Nitric oxide used to be similarly known as a poison.
But it turns out nitric oxide has a lot of vital uses in the body.
Nitric oxide opens up the arteries quickly and allows us to recover from the shock almost immediately.
It’s especially useful when we don’t have enough carbon dioxide.
Well now, here comes carbon monoxide.
We all know the carbon monoxide is a deadly poison, right?
That’s true.
But it turns out the carbon monoxide is produced in the body and is a very important ingredient in our bodies.
So let’s look at today’s paper on carbon monoxide, bilirubin, and iron.
 First, let’s talk about heme.
First, let’s talk about heme.
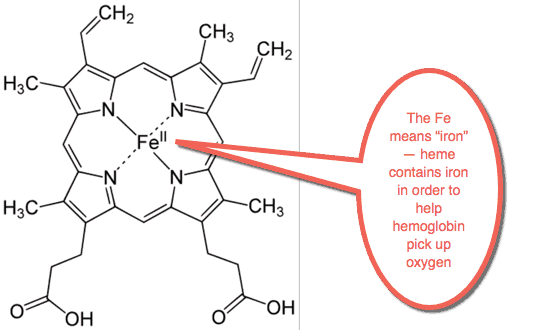
Heme is what gives blood its red color.
It’s what gives red meat its red color.
Heme assists the blood in picking up oxygen.
It also helps in transporting carbon dioxide around the body.
Heme contains at least one atom of iron in it.
Heme is probably the biggest use of iron in the body.
 But iron is very problematic.
But iron is very problematic.
Iron is the biggest source of free radicals in the human body.
High amounts of iron build up in the aging body.
This iron buildup accelerates the aging process.
People with Alzheimer’s have high amounts of iron in their brains.
But we do need iron in order to be healthy.
So iron has to be handled very carefully.
Our bodies are ingenious in handling it.
The body has to reuse heme and recycle the iron.
And in doing so, the body has to handle the iron without the iron creating huge amounts of free radicals and poisoning the body.
When heme is recycled, it first becomes bilirubin.
But in that process, carbon monoxide is produced.
And that carbon monoxide is thought to have extremely protective jobs in the human body.
Carbon monoxide shares some of the chemical and biological properties of nitric oxide, and plays a role as a widespread signal transduction mechanism for the regulation of cell function and communication.
And we discover that carbon monoxide helps us use potassium.
It helps us in learning, and even assists in opening up blood vessels:
In vessels of smaller diameter, carbon monoxide may play a more prominent role than nitric oxide as a mediator of blood vessel relaxation.
Carbon monoxide is first-aid to our hearts when we are having a heart attack.
It seems to help our tissues stay alive when they are suffering from a shortage of oxygen.
The state of being low in oxygen is called hypoxia.
As we age, we suffer more and more tissue hypoxia in our muscles, our organs, and in our brain.
Severe hypoxia takes place during a heart attack, and when we have congestive heart failure.
Carbon monoxide seems to give us urgent immediate protection during episodes of tissue hypoxia.
And then there is the important role of bilirubin — it’s not a waste product either.
Bilirubin is regarded today as the most powerful endogenous antioxidant substance.
Bilirubin helps protect our organs against the effects of iron and oxygen.
Recent studies demonstrated that bilirubin IS An efficient co-antioxidants for a-tocopherol.
That means a bilirubin works with vitamin E in providing a vital antioxidant function in our bodies.
Bilirubin is expelled in the bile.
Bile is a complex substance that is put out by the liver and gall bladder.
It’s used to aid digestion and get rid of waste products.
Bile is recycled unless it gets bound up in what we have eaten.
One of the benefits of fiber is that it can tie up bile.
When fiber does this, it helps the bile get expelled in the feces rather than recycled.
And, we do not want a lot of carbon monoxide or bilirubin in our bodies.
Carbon monoxide protects us against severe and urgent stress.
But we don’t want severe and urgent stress, ever.
It’s great that we have these protective mechanisms, but we really want to avoid using them.
We can monitor diabetic metabolism by measuring carbon dioxide exhaled in the breath.
Anyone undergoing metabolic stress will be creating more carbon monoxide.
The carbon monoxide can be monitored when exhaled.
And it can be used for diagnosis of metabolic disorders — including diabetes.
Bilirubin is a powerful anti-oxidant, but we don’t want to have to process too much, too fast.
We can help to keep our iron levels in check in a few ways.
We can avoid high iron foods.
Drinking coffee after a meal of red meat or liver helps, too.
And avoiding vitamin C when eating high iron meals is very helpful.
These steps will go a long way toward removing the strain of excess iron on the body.
Citations
New Physiological Importance of Two Classic Residual Products: Carbon Monoxide and Bilirubin
http://www.sciencedirect.com/science/article/pii/S1077315097926107
Photo courtesy of
https://www.flickr.com/photos/treehouse1977/
Exhaled Carbon Monoxide Levels Elevated in Diabetes and Correlated With Glucose Concentration in Blood*
http://journal.publications.chestnet.org/article.aspx?articleid=1078177
See this for more on Carbon Monoxide Levels, and see more on Living Healthy, and for more information see effects of Carbon Monoxide.
