
[cmamad id=”25854″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
Claim your free gift before midnight
Today Only: Click to get our incredibly popular “Small Penis – Big In Bed” course completely free
It doesn’t matter how big your penis is – you can still pleasure a woman beyond her wildest dreams.

—-Special Message—-
Because size isn’t everything – not when you know about these techniques that show men how to make the most of what they’re packing.
In fact, once you master techniques like the “super soaker,” she’ll be more than happy with how you measure up.
She may even complain of being sore the next day – in a totally good way, of course!
Just go here now – and use the coupon code GHOST to unlock the free course
———-
How To Use Methylene Blue
The way methylene blue works is very unique… And it is further distinguished by being the only treatment that’s also a dye.
Methylene blue has been used to treat:
- Malaria
- Methemoglobinemia (a blood disorder)
- Cyanide poisoning
- Psoriasis
- Septic shock
- Depression
- Post‑traumatic stress disorder
- And as a specific antidote to the cancer treatment ifosfamide
There are two ongoing clinical trials involving methylene blue: One involves treating Alzheimer’s disease and the other post‑traumatic stress disorder.
Both studies are currently in phase 2.
Methylene blue is FDA‑approved for treating methemoglobinemia.
It was grandfathered in for this indication, as it was used in medicine before the FDA even existed.
“Methylene blue has been the therapy of choice for methemoglobinemia for some 50 years.”
Methylene blue was first synthesized in 1876 and inducted into medicine before the turn of the century.
Its earliest indication was for treating malaria, a therapy spearheaded by the famous Nobel Prize winner Paul Ehrlich in 1891.
The discovery of penicillin, a natural product of mold, in 1928 usually gets credit for commencing modern molecular medicine, yet the fully‑synthetic methylene blue rightly deserves the title.
The broad utility of methylene blue can be reduced down to around five mechanisms of action…
Under low to medium doses and normal conditions, most people will only encounter two of those.
The lowest doses of methylene blue are sufficient for its FDA‑approved role in treating methemoglobinemia, as this condition is reversed in the plasma where the drug initially distributes.
Methemoglobinemia is a condition in which too much of a person’s hemoglobin exists in the oxidized state (Fe3+).
And that makes it incapable of binding and transporting oxygen.
Methemoglobinemia can of course can be fatal under some circumstances.
Yet the redox‑active methylene blue has a penchant for acquiring electrons for hemoglobin, thereby converting it into the normal ferric state (Fe2+).
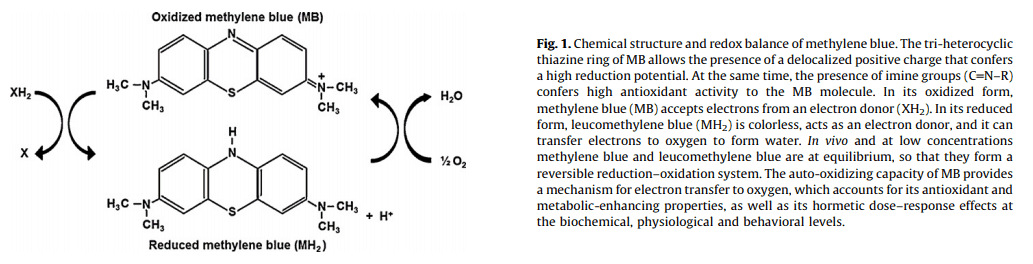
And it this effect of methylene blue – its oxidation-reduction activity – is what underlies its most common mechanism of action.
Methylene blue serves as an artificial electron carrier in the mitochondria.
It facilitates electron flow in a manner that translates into enhanced energy production.
This increases both memory and cognition at the expense of glucose and body fat.
Not only is methylene blue a redox‑active molecule (a molecule capable of easily gaining/donating electrons).
But it also resembles flavin (a biological electron transporter in the mitochondria):
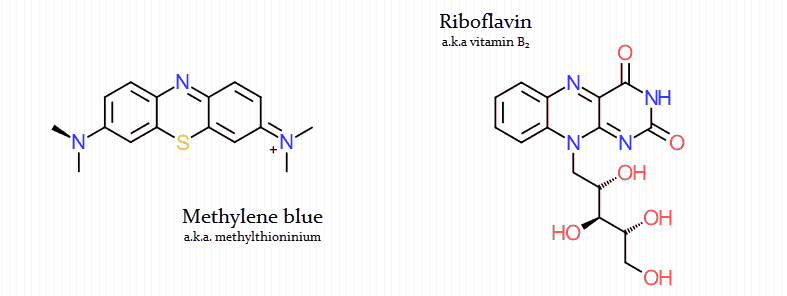
Only three molecules are primarily used biologically to transport the majority of electrons into the mitochondria:
- Flavin adenine dinucleotide (FADH2)
- Nicotinamide adenine dinucleotide (NADH)
- Coenzyme Q (CoQ)
Yet these cofactors are not very orally active, being largely incapable of crossing the cell membrane.
However, methylene blue is unique in that regard, being a molecule with notable cell permeability.
“Methylene blue has a remarkably high permeability through biomembranes, which is unparalleled by redox compounds also displaying neuroprotective properties in experimental conditions including creatine, α-lipoic acid, nicotinamide, and coenzyme Q.”
Although methylene blue is a charged molecule and is thus water‑soluble, its reduced and colorless form (leucomethylene blue) is lipid‑soluble.
The rapidity in which it cycles between both forms means that methylene blue is effectively amphiphilic – both hydrophilic (water-loving) and lipophilic (fat-loving).
So it basically exists in both solubility phases.
In this way it can float towards the cell membrane, gain two electrons, exist inside the membrane, and then donate its electrons to become cytosolic and free‑floating again.
And because its oxidized form has a positive charge, the affinity of methylene blue for the mitochondria is easy to understand.
Positive molecules are naturally attracted to mitochondria, the most electronegative organelle of the cell.
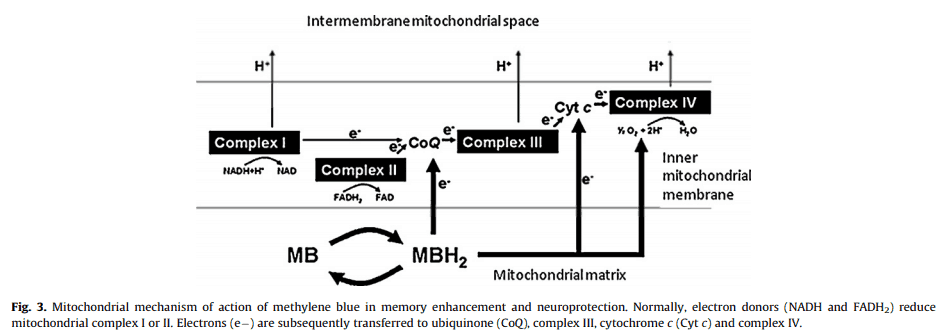
On account of its redox activity and high resemblance to flavin, methylene blue is perhaps the only molecule you’d expect to be capable of serving as its surrogate.
Methylene blue appears to be a FADH2 mimetic.
(FADH2 is a reduced form of flavin adenine dinucleotide, a redox cofactor involved in several important reactions in metabolism.)
Which may be why, at proper concentrations, methylene blue is capable of enhancing electron flow.
This was all worked out starting about 50 years ago.
But methylene blue has recently enjoyed a renewal of interest.
“It is well established that reduced methylene blue can donate electrons to coenzyme Q and possibly to cytochrome c, thus increasing cytochrome oxidase activity and oxygen consumption.”
Starting in the mid‑1990s there has been an explosion of research on methylene blue…
Dozens of animal studies have been done since then, most of which involve memory effects in rats.
Although methylene blue is still widely used in humans, studies specifically concerned with its nootropic effects are unfortunately (so far) limited to rats.
Nonetheless, there’s a good amount of useful data to be gleaned from these rat studies.
Since methylene blue works by such a fundamental mechanism, you could expect to see more or less the same effects in all mammalian species.
All cells have mitochondria, an ancient organelle that hasn’t radically changed throughout evolution.

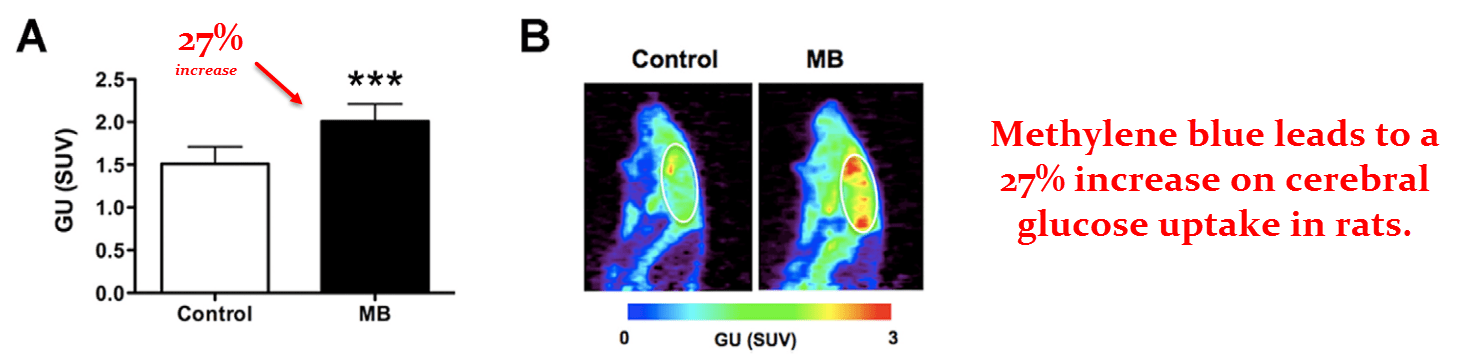
This is one of the better rat studies because it used live MRI imaging and PET scans.
These two techniques are being used to indicate how methylene blue influences cerebral oxygen (O2) and glucose consumption.
They first dosed the rats orally with .4 mg/kg of methylene blue, a conservative dose for the species.
Then they injected the rats with 0.5 mCi of [8F]‑fluorodeoxyglucose – a radioactive glucose derivative that shows up on PET scans.
This allowed them to visualize brain glucose uptake in real time to determine whatever effect methylene blue may have.
They found a 27% increase in brain glucose uptake in the group given .5 mg/kg of methylene blue.
It was, of course, a bit higher in some regions than in others.
It has been claimed that methylene blue disperses to the more active regions of the brain where it’s needed the most.
This is because, as a positively charged ion, it would be attracted to highly active mitochondria on account of greater negative charge (a higher mitochondrial membrane potential).
These researchers had also used magnetic resonance imaging (MRI) to estimate blood flow, which also increased.
The global change in blood flow was calculated to be an 18% increase the methylene blue group.
But, as with glucose uptake, this was somewhat region‑dependent.
The frontoparietal region of the cortex (a region is very important to cognition) showed the greatest change…
But blood flow also increased to all regions analyzed.
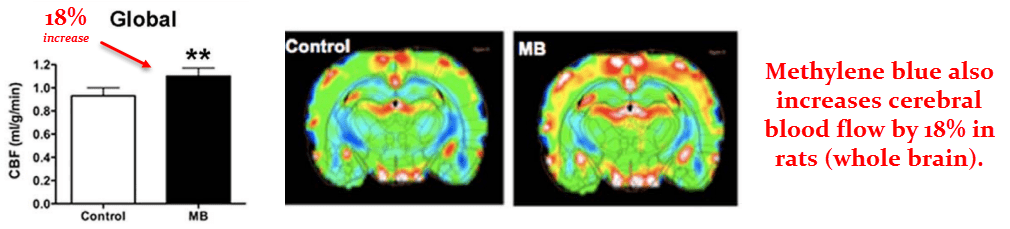
They also used two O2 sensors to measure blood oxygen levels, one placed in the femoral artery and one in the jugular vein.
They used this data to calculate the cerebral metabolic rate with respect to oxygen (O2), which increased by 39% in the methylene blue group.
It’s important to note that the percentage of blood oxygen hadn’t changed.
Methylene blue’s ability to reduce hemoglobin had been hypothesized to underlie its learning improvement effects in the past.
But experimental evidence always fails to support this idea.
Methylene blue does reduce hemoglobin. And that is the reason it’s used for treating methemoglobinemia…
But normal people don’t have much oxidized heme, so its ability to increase blood oxygen capacity is limited to certain cases.
“…Martinez Jr. et al. hypothesized that low-dose MB could facilitate memory by oxidizing methemoglobin and thereby increasing the concentration of hemoglobin with oxygen-carrying capacity.”
These researchers also demonstrated an increase in cytochrome c oxidase activity…
So it’s fair to assume that enhanced electron transport is truly methylene blue’s mechanism of action.
[cmamad id=”25855″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
The changes observed in these three metabolic parameters can account for the success of methylene blue in learning studies…
All the studies demonstrate a beneficial effect in the 1-4 mg/kg oral dose range.
There are many memory studies to choose from, and I prefer the one below as it undeniably measures a true function of memory:

This study used the 1 mg/kg dose and a 3 × 3 grid of food‑baited holes as the test.
The holes were large enough for the rodents to poke their noses into and eat out of, and also spaced far enough apart for them to navigate around each one.
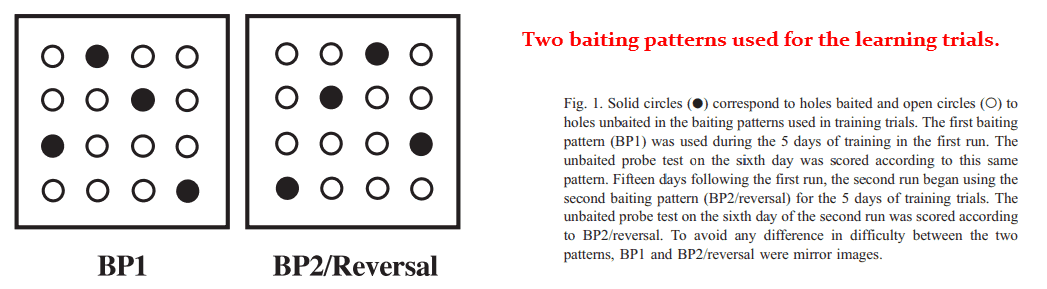
Memory was quantified by the rats’ ability to remember which holes are usually baited from day‑to‑day, a pattern which had been kept constant for weeks.
After 15 days, they reversed the pattern and graded the rats on how quickly they could adapt to the new format.
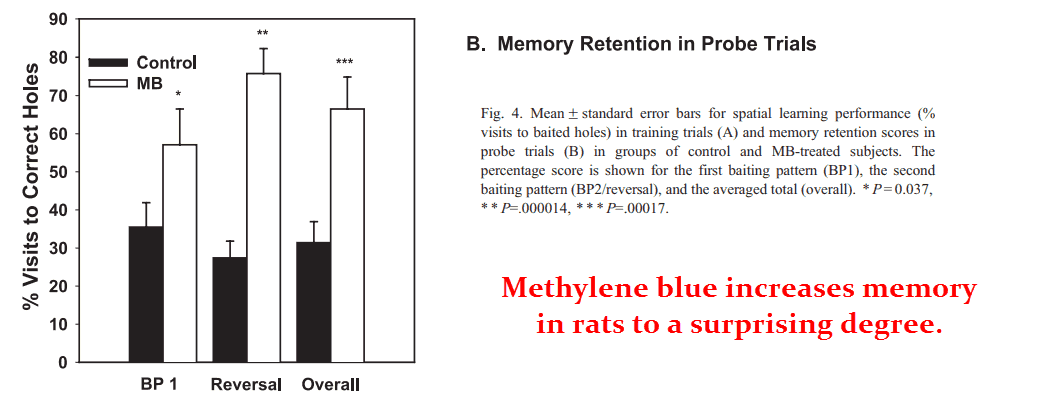
The methylene blue rats outperformed the control rats in all aspects, demonstrating a far greater capacity to learn by committing fewer errors.
The learned the initial baiting pattern faster than the control rats.
AND they also adapted to the new pattern faster.
As with most studies of this type (such as the first one we discussed here), Calloway’s measured cytochrome c oxidase activity in rat brain mitochondria.
He found a 30% increase in the experimental group.
That effect is equivalent to in vitro cellular methylene blue concentrations of .5 μM.
This is a good target concentration… In fact, with anything much higher the effect begins to decline.
“Together the findings suggest that low-dose methylene blue enhances spatial memory retention in normal rats by increasing brain cytochrome c oxidase activity.”
And very high doses actually lead to a reduced cytochrome c oxidase activity compared to controls, and also to memory inhibition in learning trials.
For this reason, proper dosing is important…
Like most drugs, methylene blue also follows an inverted U‑shaped concentration curve.
Yet extrapolating the proper human dose isn’t all that straightforward in this case, as methylene blue exhibits extensive biliary excretion in some species.
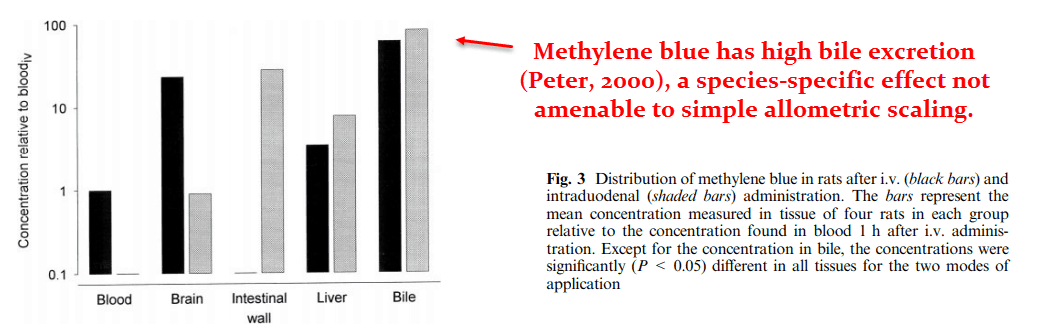
Dogs, rats, and mice are considered “good biliary excreters” – while humans and rabbits are considered “poor” ones.
Cats are considered an intermediate case, yet aren’t commonly used in pharmacokinetic studies.
In terms of phospholipid excretion, dogs excrete bile 5.56 times faster than humans per kg of body weight.
This is probably why they are said not to “absorb” methylene blue to any great extent…
And that effect is really only apparent as it is actually lost during first‑pass metabolism.
“…the fraction of dose absorbed from the gastrointestinal lumen for a large variety of drugs is remarkably consistent between animal species and humans. The bioavailability, however, differs substantially among species, presumably as a result of species differences in the magnitude of first-pass metabolism.”
It has been shown that despite a substantial 15 mg/kg oral dose, dogs will only excrete 2.4% in their urine.
Compare this with the approximately 72% bioavailability consistently measured for humans.
Simple allometric scaling of the 1 mg/kg rat dose yields a human dose around 180 micrograms/kg (μg/kg)…
Yet this may have to be reduced further to account for species‑specific biliary excretion.
Alternatively, we could back‑calculate based on:
- .5 μM concentration needed for efficient in vitro cytochrome c oxidase activity in brain cells (Callaway, 2004)
- Approximately a 1∶12 distribution ratio between the plasma and brain (Peter, 2000)
- Published human plasma levels
This calculation arrives at a dose of around 20 mg total for humans.
Yet methylene blue is a very safe treatment and there’s little risk in taking more.
Three hundred milligrams has been given for two years to depressed patients with no side effects…
And it’s currently being used right now at 260 mg in the clinical trial on post‑traumatic stress disorder (NCT01188694).
“In one notable case report (entitled “Dyed but not Dead”) published 25 years ago, a four-year old child was given a dose that was 16 times the recommended maximum. The child’s skin was intensely blue but there were no other documented ill effects.”
Methylene blue is indicated for treating depression because it acts as a monoamine oxidase inhibitor (MAOI) at very high doses (15 mg/kg in rats)…
This effect invariably leads to increased brain serotonin.
Of course, increased serotonin should be avoided. And maybe this is part of the reason why the higher doses actually impair learning in rat trials.
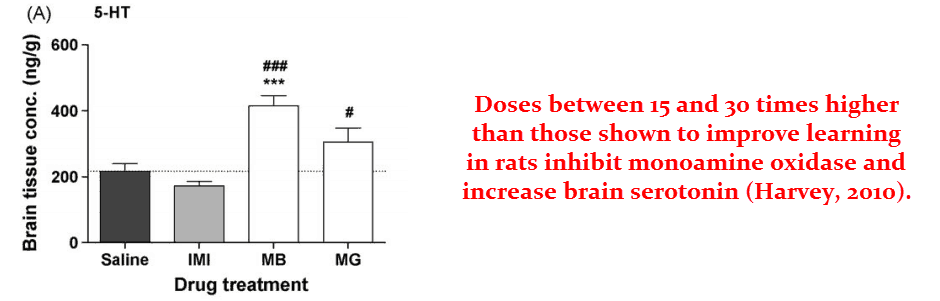
And methylene has yet another mechanism, one that occurs in the medium concentration range.
At a concentration of only 1.05 μM, methylene blue has been shown to inhibit acetylcholinesterase in human serum by 50%.
This concentration is reached by taking exactly 100 mg in humans.
To summarize methylene blue’s effect at different doses:
- Small doses (.1-20 mg) – mitochondrial metabolic enhancer
- Intermediate doses (50-100 mg) – acetylcholinesterase inhibitor
- Massive doses (> 300 mg) – monoamine oxidase inhibitor; its metabolic effects also become annulled
“Methylene blue is a reduction-oxidation (redox) agent that improves mitochondrial respiration at low concentrations (0.5-2 μM) by shuttling electrons to oxygen in the electron transport chain, thereby increasing cellular oxygen consumption.”
—-Important Message—-
Take this quick quiz
Thousands of men are taking this pop quiz and are reportedly “shocked” by their results…
Yet it is completely backed by solid studies.
———-

- Lin, Ai-Ling. "Methylene blue as a cerebral metabolic and hemodynamic enhancer." PloS one (2012)
https://www.scienceopen.com/document_file/11d8131e-3406-4aca-b04f-8988e18ee255/PubMedCentral/11d8131e-3406-4aca-b04f-8988e18ee255.pdf - Callaway, Narriman. "Methylene blue improves brain oxidative metabolism and memory retention in rats." Pharmacology Biochemistry and Behavior (2004)
https://www.sciencedirect.com/science/article/pii/S0091305703003186 - Disanto, Anthony. "Pharmacokinetics of highly ionized drugs II: methylene blue—absorption, metabolism, and excretion in man and dog after oral administration." Journal of pharmaceutical sciences (1972)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3326916/ - Antihyperglycemic, antioxidant and hypolipidemic effect of Punica granatum L flower extract in streptozotocin induced diabetic rats
https://onlinelibrary.wiley.com/doi/abs/10.1002/jps.2600610710 - Pfaffendorf, M. "The interaction between methylene blue and the cholinergic system." British journal of pharmacology (1997)
https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1038/sj.bjp.0701355 - Harvey, Brian H., et al. "Role of monoamine oxidase, nitric oxide synthase and regional brain monoamines in the antidepressant-like effects of methylene blue and selected structural analogues." Biochemical pharmacology 80.10 (2010): 1580-1591.
https://s3.amazonaws.com/academia.edu.documents/45819519/j.bcp.2010.07.03720160520-17782-5t7oht.pdf?AWSAccessKeyId=AKIAIWOWYYGZ2Y53UL3A&Expires=1550983077&Signature=IdEOIzjGzfPPiO6j5DH48lE29GQ%3D&response-content-disposition=inline%3B%20filename%3DRole_of_monoamine_oxidase_nitric_oxide_s.pdf
- Methylene Blue
https://www.drugs.com/monograph/methylene-blue.html - Methylene Blue
http://www.academia.edu/Documents/in/Methylene_Blue
