
[cmamad id=”23089″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
Breakthrough: why the Chinese had never heard of prostate cancer for 1000 years
—-Important Message—-
For men who want to get even better erections – here’s a new 6-minute video that shows you how:
- Have a penis that looks bigger and thicker
- Banish performance anxiety for good…
- Impress her with surging erections that last 30 minutes or more…
- And perform better in bed than you have in years (maybe ever)
Watch this video to get great erections whenever you want, even if it’s been years.

———-
Baicalein – 100-year-old cancer cure (especially for prostate)
Before the age of synthetic chemistry, all drugs were extracted from plants.
Many still are: aspirin, digitoxin, caffeine, taxol, theophylline, nicotine, cocaine, atropine, and morphine…
Those are just a few natural phytochemicals commonly used today.
Although natural drugs are still occasionally being discovered in plants, finding unrealized effects of older ones is more common.
Of course, there are many examples of phyto-drugs that did not live up to their expectations…
Curcumin became popular, somehow, despite it being practically unabsorbable.
And we’ve always made far too much of resveratrol, popularized to explain the so-called “French Paradox.”
Yet this needs no explanation because saturated fats don’t “cause heart disease.”
Baicalein was isolated in the 1930s, so it isn’t new… It’s certainly not a “trend.”
Back in 1982, this molecule was found to inhibit 12-lipoxygenase – and then glyoxalase four years later.
(Those are enzymes.)
Both of these activities are surprisingly potent…
Baicalein became the gold standard research chemical used to inhibit lipoxygenase and today is still the most potent natural glyoxalase inhibitor among all natural flavonoids tested.
[cmamad id=”23090″ align=”center” tabid=”display-desktop” mobid=”display-desktop” stg=””]
More recently, it’s been shown to inhibit estrogen receptor activation in low micromolar concentrations, even though it doesn’t bind to it directly.
How exactly baicalein can exhibit such a broad spectrum of effects on so many receptors and enzymes, and at such low doses, can only be understood in one way…

We can ignore one half of that study’s title…
Valerie Randall and others have proven that androgens actually stimulate hair growth – just as true on the scalp as in the beard.
This study shows that baicalein precursor can inhibit androgen receptor activation just as well as flutamide – even though it doesn’t actually bind to it.
The inhibition was proven directly using fluorescent antibodies against the androgen receptor (green) and a DNA-binding stain for the nucleus (blue).
When the two images are superimposed, you can readily see the number of androgen receptors that have gone into the nucleus:
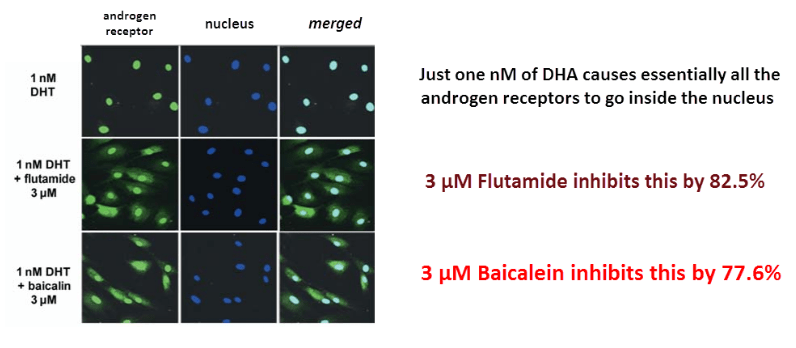
Baicalein unambiguously inhibits estrogen receptor migration.
But how? The activity of flutamide is easy to comprehend because it’s an androgen analog that powerfully binds and blocks the DHT binding site.
Baicalein doesn’t interact with the androgen receptor at all, yet it could inhibit receptor translocation just as well as flutamide – and at the same concentration.
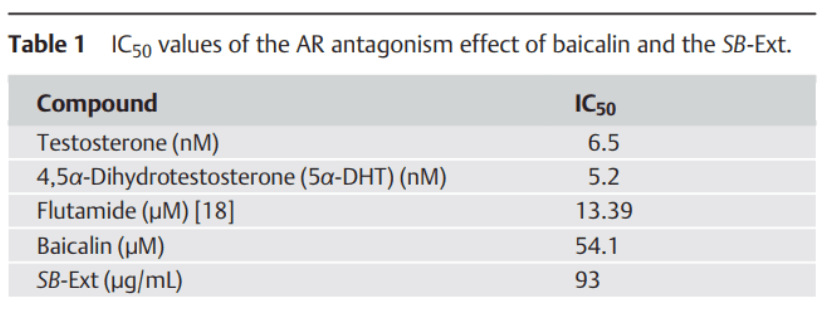
It is difficult to reconcile this powerful activity of baicalein with its low affinity for the androgen receptor:
We see the very same thing with estrogen receptors and carbonic anhydrase…
These other proteins are also affected by baicalein despite it having no affinity towards them.
Yet baicalein has been proven to bind and inhibit only one key enzyme directly – this is called glyoxalase I.
Baicalein inhibits the enzyme that converts methylglyoxal into lactic acid in low concentrations.
By doing that, it increases methylglyoxal concentrations throughout the entire cell.
This high-affinity binding had been proven through X-ray analysis:
“In this study, 16 flavonoids were examined, and only baicalein is identified as a potent in vitro glyoxalase I. X-ray crystallographic analysis reveals that baicalein chelates with the catalytic zinc ion…”
The glyoxalase enzymes regulate methylglyoxal concentrations within the cell, transforming it to lactic acid.
As mentioned above, inhibiting glyoxalase leads to increased methylglyoxal concentrations.
In 2007, Yao Dashun showed that methylglyoxal can modify the transcription factor Sp3, thereby facilitating transcription of Sp1 by eliminating its competitor.
(SP3 and SP1 refer to specific proteins and the specific genes that encode them.)
That was the first physical proof that methylglyoxal influences gene transcription…
Although scientists knew about its powerful anti-cancer effects for nearly 100 years.
They also proved exactly how it does this.
Methylglyoxal freely adds to arginine side chains in its classic reaction, thereby transforming them into hydroimidazolone side chains:
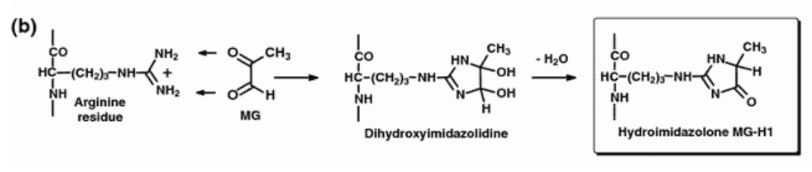
The cellular methylglyoxal concentration appears capable of causing cell-wide effects this way, by modifying key arginines on enzymes and receptors.
Methylglyoxal is a normal product of glucose metabolism used by the cell to sense and respond to its glycolytic rate in real time.
Baicalein can increase methylglyoxal concentrations as reliably as just about anything, perfectly explaining its nonspecific yet specific effects.
Any other explanation fails horribly…
You would have to assume that baicalein actually binds all the receptors and enzymes it inhibits:
(1) The estrogen receptor
(2) The androgen receptor
(3) Lipoxygenase
(4) Carbonic anhydrase
It strongly affects all of these in micromolar concentrations.
Baicalein increases methylglyoxal more powerfully than just about anything… It’s on par with the infamous lapachol.
Yet, unlike lapachol and beta-lapachol, you can easily buy baicalein in crystalline form.
All four proteins modified by baicalein contain key arginines…
The estrogen receptor, the androgen receptor, carbonic anhydrase, and 12-lipoxygenase all have key arginines in their binding/catalytic domains.
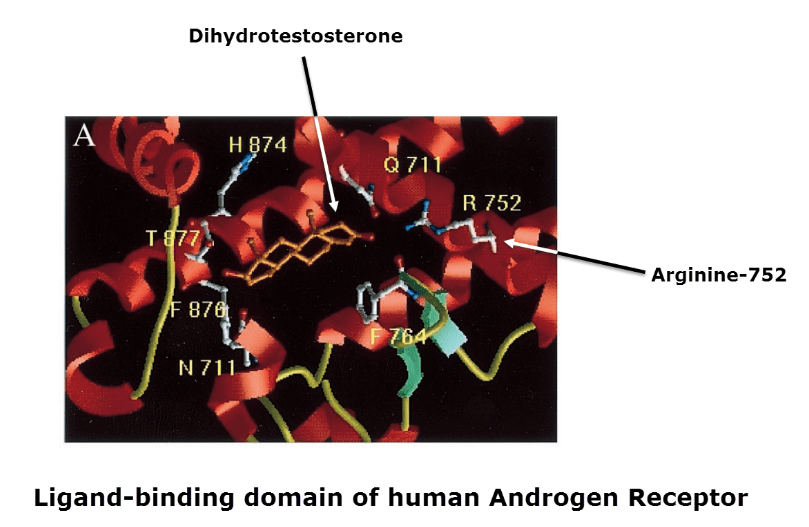
Arginine-752 is indispensable for binding DHT/testosterone at the 3-keto group.
This is proven by human and rat polymorphisms showing how a change in this amino acid leads to androgen insensitivity.
Arginine-752 is analogous with Arginine-394 of the estrogen receptor, the amino acid that binds to estradiol’s 3-hydroxy group.
The dependency of steroid-arginine binding is probably why baicalein inhibits them both in the same way.
I think we could expect methylglyoxal to do likewise.
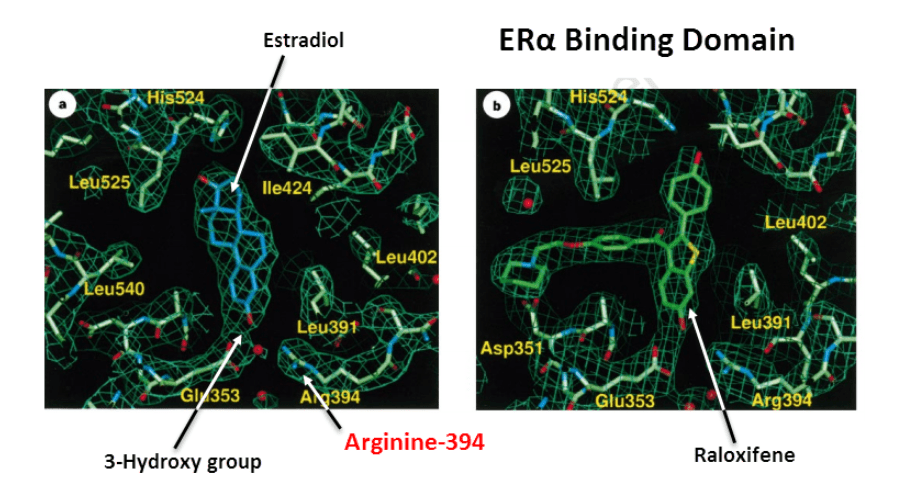
Baicalein does not bind to the receptor. Nor does it inhibit aromatase.
Nonetheless, baicalein reduces estrogen receptor alpha translocation in very low concentrations.
As we said above, lipoxygenase was the first enzyme shown to be inhibited by baicalein – even before glyoxalase…
And the arginine at the entrance to its catalytic domain is so important that it’s been given a special name: the “Hornung determinant.”
The Hornung determinant is an exposed methylglyoxal target and explains baicalein’s effect perfectly.
“It is suggested that the presence of the smaller amino acid demasks a positively charged arginine residue, the so-called ‘Hornung determinant’, at the bottom of the substrate pocket…”
This key arginine side chain (the “Hornung determinant”) is thought to bind linoleic acid, the enzyme’s substrate, using its inherent positive charge to grab the fatty acid’s negative end.
Lipoxygenase oxidizes linoleic acid, unbelievably, into an even more carcinogenic form.
The indispensable arginine is a characteristic feature of all lipoxygenase enzymes.
The proper functioning of 12-lipoxygenase appears impossible after its arginine is methyl-glyoxalated.
Just as with the two steroid receptors above, baicalein must be acting through a methylglyoxal intermediate.
“This guanidino group may then be able to form a salt bridge with the carboxylic group of the substrate, favoring an inverse head-to-tail substrate orientation. This special role of this arginine residue may also be supported by the fact that it is highly conserved in plant lipoxygenases.”
Lipoxygenase mRNA expression is correlated with prostate cancer and also with its severity.
Besides glyoxalase I and lipoxygenase, another enzyme is highly sensitive to methylglyoxal concentrations: Carbonic anhydrase.
Carbonic anhydrase is the fastest known enzyme.
It operates roughly 200,000 times per second.
Since CO₂ is the main product of sugar metabolism, you’d hope this enzyme could sense glucose levels if any enzyme could.
Carbonic anhydrase senses glycolysis rate via methylglyoxal, with the arginine-reactive product always proportional to it.
Methylglyoxal concentrations always increase carbonic anhydrase activity.
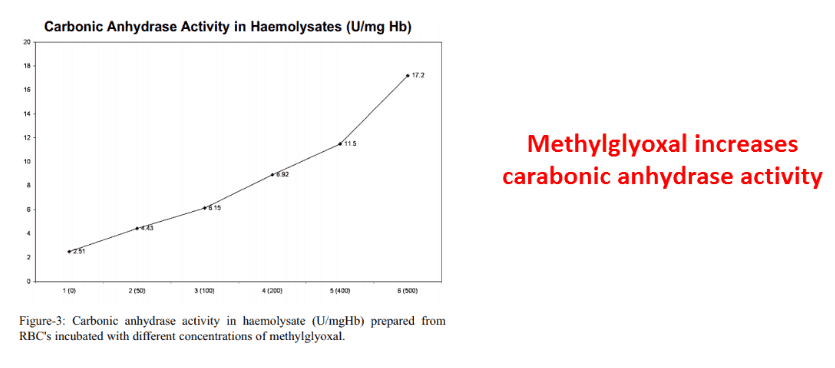
This is somewhat unique…
Most drugs that influence carbonic anhydrase act in the other direction – to inhibit.
Aspirin, acetazolamide, and the sulfonamides all act to inhibit this enzyme.
Methylglyoxal is likely working here to increase the enzyme’s catalytic rate by modifying its “proton wire” – making it more efficient.
The proton wire of carbonic anhydrase rapidly shuttles free protons away from its active zinc atom, keeping the pH in range.
The imidazole ring formed by methylglyoxal and arginine has an acid dissociation constant of around 7, meaning these molecules have some of the highest rates of proton transfer.
And proton transfer is what limits the enzyme’s rate, not carbon dioxide or water.
Methylglyoxal is the only thing besides baicalein we know of that accelerates this enzyme:
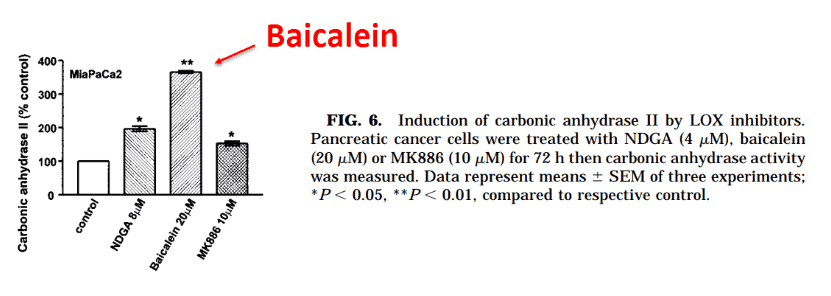
Baicalein always works through methylglyoxal, its “second messenger” – inducing universal effects by targeting certain enzymes and receptors.
Carbonic anhydrase also has a very important arginine, number 36, that is conserved across all species and subtypes.
The transformation of Arg-36 into hydroimidazolone-36 should enhance the speed of proton flow, accelerating catalysis by facilitating hydron removal.
“Arg-36 variants had both kcat and kcat/Km values that were decreased at least 250-fold relative to those of the wild type, establishing an essential function for this residue.”
(NOTE: kcat and kcat/Km are values that have to do with the catalytic efficiency of enzymes.)
It’s remarkable that every enzyme and every receptor inhibited by baicalein also has an arginine indispensable for its proper function…
The estrogen receptor has Arg-394, the androgen receptor has Arg-752, carbonic anhydrase has Arg-36, and 12-lipoxygenase has the notorious Hornung determinant.
There can be little doubt as to what is going on here…
There is simply no other way of explaining baicalein’s cell-wide effects in micromolar concentrations.
“Between these inhibitors, baicalein caused the greatest increase in carbonic anhydrase II activity…”
Out of the five enzymes and receptors influenced by baicalein, only glyoxalase has been proven to actually bind it.
And based on its flavone structure, you wouldn’t expect baicalein to even bind any other.
Methylglyoxal is an underappreciated “second messenger” – the fundamental glycolysis signal used by all living cells, from bacteria to vertebrates.
Baicalein, lapachol, and beta-lapachone are the three most potent natural glyoxalase I inhibitors…
They are capable of doubling methylglyoxal concentrations in achievable concentrations.
—-Important Message—-
WARNING: Are you taking one of these 5 blood pressure drugs?
Has your doctor warned you about these blood pressure treatments yet?
The FDA is trying to say they’re “okay” to take short-term, but why would you ever want to take something that could give you cancer?
Not for me, no thank you.
———-

- Kim, Ah-Reum. "The inhibitory effect of Scutellaria baicalensis extract and its active compound, baicalin, on the translocation of the androgen receptor with implications for preventing androgenetic alopecia." Planta medica (2014) https://www.ncbi.nlm.nih.gov/pubmedj/24496985
- Iio, Masayoshi. "Effects of baicalein, a flavonoid, and other anti-inflammatory agents on glyoxalase-I activity." Agricultural and biological chemistry (1986) https://www.tandfonline.com/doi/pdf/10.1080/00021369.1986.10867521
- Yao, Dachun. "High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A." Journal of Biological Chemistry (2007) http://www.jbc.org/content/282/42/31038.full.pdf
- Po, Lai See. "Baicalein and genistein display differential actions on estrogen receptor (ER) transactivation and apoptosis in MCF-7 cells." Cancer letters (2002) https://www.sciencedirect.com/science/article/pii/S0304383502003555
- Zhang, Hong. "In Vitro Inhibition of Glyoxalase by Flavonoids: New Insights from Crystallographic Analysis." Current topics in medicinal chemistry (2016) https://www.researchgate.net/profile/Xiaopeng_Hu/publication/281057604_In_Vitro_Inhibition_of_Glyoxalase_I_by_Flavonoids_New_Insights_from_Crystallographic_Analysis/links/5854425808ae77ec37045b3a/In-Vitro-Inhibition-of-Glyoxalase-I-by-Flavonoids-New-Insights-from-Crystallographic-Analysis.pdf
- Baicalein – An Intriguing Therapeutic Phytochemical in Pancreatic Cancer https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3678518/
- Baicalein, a novel anti-diabetic compound - VTechWorks - Virginia Tech https://vtechworks.lib.vt.edu/handle/10919/76854
